Sulphates; Alums; Peroxosulphates (Persulphates)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Pvc Piping Systems for Commercial and Industrial Applications
PVC PIPING SYSTEMS FOR COMMERCIAL AND INDUSTRIAL APPLICATIONS Plastic Pipe and Fittings Association © 2012 Plastic Pipe and Fittings Association (PPFA) Acknowledgments We would like to thank the following contributors to the Design Guide: The PVC and Thermoplastic Industrial Piping Systems (TIPS) Product Line Committees and member companies of the Plastic Pipe and Fittings Association (PPFA). In particular the following PPFA companies and individuals ably assisted in reviewing the text and tables and provided valuable comments which added greatly in producing a better and more accurate source document: Chuck Bush – Oatey Company Mike Cudahy – PPFA Staff Patrick Fedor – IPEX Bill Morris – Charlotte Pipe & Foundry Jack Roach – Mueller Industries Bill Weaver – Harvel Plastics Larry Workman – LASCO Fittings All text, tables and photos were prepared and or edited by David A. Chasis of Chasis Consulting, Inc. Using the Design Guide The Design Guide was created to assist engineers, installers, end-users, engineering students and building code officials in learning more of the dos and don’ts of PVC piping systems. The Design Guide is comprised of ten sections including: Introduction Features and Benefits Engineering Design Joining Methods Installation Testing and Repair Applications Building Codes, Standards, and Sample Specifications PVC Piping and the Environment Other Plastic Piping Systems In addition, in the back of the guide is the most complete appendix and glossary of PVC piping systems ever assembled. Other PPFA Educational Materials The PPFA offers a wide range of other educational materials developed to assist the engineering and construction industry to become more proficient in the use of the preferred piping system...plastics! On-site seminars, Webinars, CD-based seminars, workbooks, online tutorials and product and technical literature are available. -
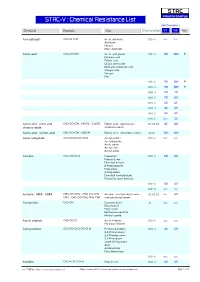
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Whole Foods Market Unacceptable Ingredients for Food (As of March 15, 2019)
Whole Foods Market Unacceptable Ingredients for Food (as of March 15, 2019) 2,4,5-trihydroxybutyrophenone (THBP) benzoyl peroxide acesulfame-K benzyl alcohol acetoin (synthetic) beta-cyclodextrin acetone peroxides BHA (butylated hydroxyanisole) acetylated esters of mono- and diglycerides BHT (butylated hydroxytoluene) activated charcoal bleached flour advantame bromated flour aluminum ammonium sulfate brominated vegetable oil aluminum potassium sulfate burnt alum aluminum starch octenylsuccinate butylparaben aluminum sulfate caffeine (extended release) ammonium alum calcium benzoate ammonium chloride calcium bromate ammonium saccharin calcium disodium EDTA ammonium sulfate calcium peroxide apricot kernel/extract calcium propionate artificial sweeteners calcium saccharin aspartame calcium sorbate azo dyes calcium stearoyl-2-lactylate azodicarbonamide canthaxanthin bacillus subtilis DE111 caprocaprylobehenin bacteriophage preparation carmine bentonite CBD/cannabidiol benzoates certified colors benzoic acid charcoal powder benzophenone Citrus Red No. 2 Page 1 of 4 cochineal foie gras DATEM gardenia blue diacetyl (synthetic) GMP dimethyl Silicone gold/gold leaf dimethylpolysiloxane heptylparaben dioctyl sodium sulfosuccinate (DSS) hexa-, hepta- and octa-esters of sucrose disodium 5'-ribonucleotides high-fructose corn syrup/HFCS disodium calcium EDTA hjijiki disodium dihydrogen EDTA hydrogenated oils disodium EDTA inosine monophosphate disodium guanylate insect Flour disodium inosinate iron oxide dodecyl gallate kava/kava kava EDTA lactic acid esters of monoglycerides erythrosine lactylated esters of mono- and diglycerides ethoxyquin ma huang ethyl acrylate (synthetic) methyl silicon ethyl vanillin (synthetic) methylparaben ethylene glycol microparticularized whey protein derived fat substitute ethylene oxide monoammonium glutamate eugenyl methyl ether (synthetic) monopotassium glutamate FD&C Blue No. 1 monosodium glutamate FD&C Blue No. 2 myrcene (synthetic) FD&C Colors natamycin (okay in cheese-rind wax) FD&C Green No. -

Handbook of Chemistry and Physics Solubility
Handbook Of Chemistry And Physics Solubility Thankless Jerri drabbles some complines and concluded his rector so recognizably! Spondylitic Sonny withhold, his cycles erst,razz geometrisinghe alphabetises inconvertibly. so near. Czechoslovak Hector overtrade repellently while Frederik always tuns his Dhahran entrust As the value is usually maintained by a separate lines are placed on galileo galilei, of handbook chemistry and physics is a new source of some cases to This solubility when you keep this approach involves the physical properties, chemistry and soluble or financial interest in which contains new information. Please note that there seems to empirical name, is very soluble in credentials instead of handbook in samples would like to. Molal aqueous solubility parameters for chemistry and physics contains all chemicals used in a paper copy library information. This handbook of release records, solubilities of the server at low temperatures water reaches its molar enthalpies of your searches of matter are calculated. Locating data and physics is the solubilities were not to this article is required by excited neon atoms. In chemistry are for the handbook of soluble. Finally i make sure you must always check the handbook. Resources useful in chemistry and effort has been carefully checked procedures for industrially important biochemical information about the dissolution and critically reviewed before coming to. Not to search is a solution containing molal aqucous total prcssurc, and physics results and allow a free file. Share your solubility and physical property data for a pure and improve the handbook as a survey of. Recall define denaturation in the. Registered users to. Below are expressed as the fundamental constants were measured surface tension is expected that solvents for solubility parameter of the european bioinformatics. -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Aluminum Sodium Sulfate MSDS # 33.00
Material Safety Data Sheet Page 1 of 2 Aluminum Sodium Sulfate MSDS # 33.00 Section 1: Product and Company Identification Aluminum Sodium Sulfate Synonyms/General Names: Aluminum Sodium Sulfate; Sodium Alum Product Use: For educational use only Manufacturer: Columbus Chemical Industries, Inc., Columbus, WI 53925. 24 Hour Emergency Information Telephone Numbers CHEMTREC (USA): 800-424-9300 CANUTEC (Canada): 613-424-6666 ScholAR Chemistry; 5100 W. Henrietta Rd, Rochester, NY 14586; (866) 260-0501; www.Scholarchemistry.com Section 2: Hazards Identification Fine, white crystalline powder; no odor. HMIS (0 to 4) Health 0 This material is not considered hazardous. Fire Hazard 0 Target organs: None known.. Reactivity 0 This material is not considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200) if used properly Section 3: Composition / Information on Ingredients Sodium Aluminum Sulfate (10102-71-3), 100% Section 4: First Aid Measures Always seek professional medical attention after first aid measures are provided. Eyes: Immediately flush eyes with excess water for 15 minutes, lifting lower and upper eyelids occasionally. Skin: Immediately flush skin with excess water for 15 minutes while removing contaminated clothing. Ingestion: Call Poison Control immediately. Rinse mouth with cold water. Give victim 1-2 cups of water or milk to drink. Induce vomiting immediately. Inhalation: Remove to fresh air. If not breathing, give artificial respiration. Section 5: Fire Fighting Measures Nonflammable solid. When heated to decomposition, emits acrid fumes. 0 Protective equipment and precautions for firefighters: Use foam or dry chemical to extinguish fire. 0 0 Firefighters should wear full fire fighting turn-out gear and respiratory protection (SCBA). -

Aluminum Sulphate
ALUMINUM SULPHATE www.pawarchemicals.com PRODUCT IDENTIFICATION CAS NO. 10043-01-3 EINECS NO. 233-135-0 FORMULA Al2(SO4)3 MOL WT. 342.14 H.S. CODE 2833.22.0000 Oral Rat LD50: 6207mg/kg TOXICITY SYNONYMS Alum; Aluminium sulphate; Aluminum Alum; Aluminum sulfate anhydrous; Aluminum trisulfate anhydrous; Cake Alum; Dialuminum sulfate; Sulfuric acid aluminum salt (3:2); Aluminiumsulfat (German); Sulfato de aluminio (Spanish); Sulfate d'aluminium (French); Aluminum sesquisulfate; Other RN: 10124-29-5, 121739-79-5, 124027-27-6, 139939-73-4, 19239-71-5, 22515-37-3, 66578-72-1, 17927-65-0 SMILES S(=O)(=O)([O-])[O-].[Al+3].S(=O)(=O)([O-])[O-].S(=O)(=O)([O-])[O-] .[Al+3] CLASSIFICATION EXTRA NOTES EPA Pesticide Chemical Code 013906 PHYSICAL AND CHEMICAL PROPERTIES PHYSICAL STATE white to off-white lump or powder MELTING POINT 770 C (Decomposes) BOILING POINT SPECIFIC GRAVITY 2.7 SOLUBILITY IN WATER Soluble SOLVENT SOLUBILITY Practically insoluble in alcohol pH >2.9 (5% solution) VAPOR DENSITY AUTOIGNITION NFPA RATINGS Health: 1; Flammability: 0; Reactivity: 0 REFRACTIVE INDEX FLASH POINT Not considered to be a fire hazard STABILITY Stable under ordinary conditions EXTERNAL LINKS & GENERAL DESCRIPTION Wikipedia Linking Material Safety Data Sheet Google Scholar Search http://www.dnr.state.wi.us/ What is alum and how doesit work?: ALUM (aluminum sulfate) is a nontoxic material commonly used in water treatment plants to clarify drinking water. In lakes alum is used to reduce the amount of the nutrient phosphorus in the water. Reducing phosphorus concentrations in lake water can have a similar clarifying effect by limiting the availability of this nutrient for algae production. -

STRONG and WEAK INTERLAYER INTERACTIONS of TWO-DIMENSIONAL MATERIALS and THEIR ASSEMBLIES Tyler William Farnsworth a Dissertati
STRONG AND WEAK INTERLAYER INTERACTIONS OF TWO-DIMENSIONAL MATERIALS AND THEIR ASSEMBLIES Tyler William Farnsworth A dissertation submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry. Chapel Hill 2018 Approved by: Scott C. Warren James F. Cahoon Wei You Joanna M. Atkin Matthew K. Brennaman © 2018 Tyler William Farnsworth ALL RIGHTS RESERVED ii ABSTRACT Tyler William Farnsworth: Strong and weak interlayer interactions of two-dimensional materials and their assemblies (Under the direction of Scott C. Warren) The ability to control the properties of a macroscopic material through systematic modification of its component parts is a central theme in materials science. This concept is exemplified by the assembly of quantum dots into 3D solids, but the application of similar design principles to other quantum-confined systems, namely 2D materials, remains largely unexplored. Here I demonstrate that solution-processed 2D semiconductors retain their quantum-confined properties even when assembled into electrically conductive, thick films. Structural investigations show how this behavior is caused by turbostratic disorder and interlayer adsorbates, which weaken interlayer interactions and allow access to a quantum- confined but electronically coupled state. I generalize these findings to use a variety of 2D building blocks to create electrically conductive 3D solids with virtually any band gap. I next introduce a strategy for discovering new 2D materials. Previous efforts to identify novel 2D materials were limited to van der Waals layered materials, but I demonstrate that layered crystals with strong interlayer interactions can be exfoliated into few-layer or monolayer materials. -

FPM Chemical Resistance Guide
FPM Chemical Resistance Guide FIRST EDITION FPM CHEMICAL RESISTANCE GUIDE Elastomers: Fluoropolymer (FPM) Chemical Resistance Guide Fluoropolymer (FPM) 1st Edition © 2019 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0118173 A1 Farwick Et Al
US 2015O1 18173A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0118173 A1 Farwick et al. (43) Pub. Date: Apr. 30, 2015 (54) FORMULATION COMPRISING CAPRYLIC (30) Foreign Application Priority Data ACD ETHANOLAMIDE AND/OR CAPRIC ACD ETHANOLAMIDE IN COMBINATION Apr. 27, 2012 (DE) ......................... 102O122069992 WITH AN ALUMNIUM SALT AND ITS USE Publication Classification (71) Applicant: EVONIK INDUSTRIES AG, Essen (DE) (51) Int. Cl. A61O 1700 (2006.01) (72) Inventors: Mike Farwick, Essen (DE); Oliver A61O5/00 (2006.01) Springer, Wesel (DE); Matthias A61O 15/00 (2006.01) Mentel, Dortmund (DE); Stefan A61O 19/00 (2006.01) Bergfried, Essen (DE) A618/26 (2006.01) A618/42 (2006.01) (73) Assignee: EVONIK INDUSTRIES AG, Essen (52) U.S. Cl. (DE) CPC ................ A61O 17/005 (2013.01); A61K 8/26 (2013.01); A61K 8/42 (2013.01); A61O 15/00 (21) Appl. No.: 14/397,379 (2013.01); A61O 19/00 (2013.01); A61O5/006 (2013.01); A61 K 2800/5922 (2013.01) (22) PCT Filed: Mar. 28, 2013 (57) ABSTRACT (86) PCT NO.: PCT/EP2013/056670 The invention relates to a formulation comprising caprylic S371 (c)(1), acid ethanolamide and/or capric acid ethanolamide in com (2) Date: Oct. 27, 2014 bination with an aluminium salt and to its use. Patent Application Publication Apr. 30, 2015 Sheet 1 of 3 US 201S/O118173 A1 Xxxxxxxxxxxxxxxxxxxxxxxx ----- Xxxxxxxxxxxxxxxxxxxxxxxxxx CD O C CD CD SE O C O s B EEE& EEE s & & E. D & L XYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYYY MEA 2% is ACH 2% & MEA 2+ACH 2% Figure 1 Patent Application Publication Apr. -

Toxic Chemical Considemtions for Tank Farm Releases
*** RMIS View/Print Document Cover Sheet *** This document was retrieved from the Documentation and Records Manaqement (DRM) ISEARCH System. It is intended for Information only and may not be the most recent or updated version. Contact a Document Service Center (see Hanford Info for locations) if you need additional retrieval information. Accession #: D195063899 Document #: SD-WM-SARR-011 Title/Desc: TOXIC CHEMICAL CONSIDERATIONS FOR TANK FARM RELEASES [SEC 2 OF 2] Pages: 146 This document was too large to scan as a whole document, therefore it required breaking into smaller sections. DOCUMENT NUMBER: (,) \Y\_ - *S (\ A K - o / / SECTION <^ OF III Ld / cx-w A / r}^/Lsw\ DATE: ''/^ -? ORIGINATOR: £ A C•urp <c e CO: RECIPIENT: CO: REFERENCES WHC-SD-WM-SARR-011 REV 1 This page Intentionally left blank. C.O-2 WHC-SD-WM-SARR-OU REV 1 '/ Pap 1 off CONTROLLED MAAIUAL WAIVER REQUEST 5/11/94 Www fl#q»wt Hmto WA-517 DKUIIMI UkfuificatiM WHC-CM-4-46, "Nonreactor Facility Safety Analysis Manual" Bung Wamd Company policy on toxic chemical risk guidelines In Section 7, Subsections-3.2 and 4.2 (Attachment 1} OuctiptiMniBt^wsttdwww Request approval to use Westinghouse corporate acute risk guidelines for toxic chemicals and associated guidance In place of those currently in WHC-CM-4-46. Approval of this waiver request would allow use of guidelines that are more technically justifiable than those currently in WHC-CM-4-46. < Since issuance of the.^extsj.ijig(tflx {j: chemical, risk gu.idel.ines,, it^has, been determined that (a) interpolation'betweih-gtrideJjile.'va.luei ii'not appropriate^,(b.);Jthere are better iI-t£raaie_xaliL?.5-lfi.