Anterior Chest Wall in SAPHO Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diapositiva 1
Thoracic Cage and Thoracic Inlet Professor Dr. Mario Edgar Fernández. Parts of the body The Thorax Is the part of the trunk betwen the neck and abdomen. Commonly the term chest is used as a synonym for thorax, but it is incorrect. Consisting of the thoracic cavity, its contents, and the wall that surrounds it. The thoracic cavity is divided into 3 compartments: The central mediastinus. And the right and left pulmonary cavities. Thoracic Cage The thoracic skeleton forms the osteocartilaginous thoracic cage. Anterior view. Thoracic Cage Posterior view. Summary: 1. Bones of thoracic cage: (thoracic vertebrae, ribs, and sternum). 2. Joints of thoracic cage: (intervertebral joints, costovertebral joints, and sternocostal joints) 3. Movements of thoracic wall. 4. Thoracic cage. Thoracic apertures: (superior thoracic aperture or thoracic inlet, and inferior thoracic aperture). Goals of the classes Identify and describe the bones of the thoracic cage. Identify and describe the joints of thoracic cage. Describe de thoracic cage. Describe the thoracic inlet and identify the structures passing through. Vertebral Column or Spine 7 cervical. 12 thoracic. 5 lumbar. 5 sacral 3-4 coccygeal Vertebrae That bones are irregular, 33 in number, and received the names acording to the position which they occupy. The vertebrae in the upper 3 regions of spine are separate throughout the whole of life, but in sacral anda coccygeal regions are in the adult firmly united in 2 differents bones: sacrum and coccyx. Thoracic vertebrae Each vertebrae consist of 2 essential parts: An anterior solid segment: vertebral body. The arch is posterior an formed of 2 pedicles, 2 laminae supporting 7 processes, and surrounding a vertebral foramen. -
Thorax Is Upper Part of Trunk •Study of Thorax by Studying: –Wall of the Thorax –Contents of Thorax Wall of Thorax
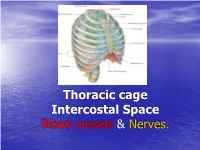
Thoracic cage Intercostal Space Blood vessels & Nerves. General Overview •Thorax is upper part of trunk •Study of thorax by studying: –Wall of the thorax –Contents of thorax Wall of thorax •Skeletal framework-Thoracic cage •Muscles, membrane & ligaments Thoracic cage Thoracic Cage • Conical in shape with truncated apex • Boundaries: • Anteriorly : Sternum • Posteriorly: Twelve Thoracic Vertebrae • On sides : Twelve pairs of Ribs & Costal Cartilages Thoracic cage • The thoracic wall is covered from outside to inside by • skin • superficial facia • deep fascia & • extrinsic muscle. • Muscles of the upper limb: • Pectoralis major Pectoralis minor • Trapezius, Serratus anterior, lattisimus dorsi • Levator scapulae, Rhomboideus major & minor • Serratus posterior superior & Inferior. • Muscles of the abdomen: • Rectus abdominis & external oblique • Muscle of the back: Erector spinae Ribs • True Ribs: articulating with sternum anteriorly – Vertebrosternal ribs: 1-7 ribs • False Ribs: Do not articulate with sternum anteriorly – Vertebrochondral ribs: 8-10 ribs – Floating ribs: 11-12 ribs Ribs First & 12th ribs Apertures of thorax • Superior Aperture (Inlet of Thorax) communicates thorax & neck. Structures passing between neck & thorax are contents of inlet. Partly covered by suprapleural membrane. • Inferior Aperture: Closed by Diaphragm. Structures passing between thorax & Abdomen pass through openings in diaphragm Joints of thorax Joints Joints Joints of thorax • Intervertebral joints: joints between bodies of vertebrae. It is Symphyseal type of joint. • Joint between articular processes of vertebrae is Plane synovial joint • Costovertebral joint: It is plane synovial joint between rib & vertebrae. • Manubriosternal joint: Between manubrium & Body of sternum, Symphyseal joint. • Xiphisternal joint: Between body of sternum & xiphoid process, symphyseal joint Joints of thorax • Sternocostal joints: – First Sternocostal joint between first rib & sternum is primary cartilaginous joint which is responsible for movement of sternum during respiration. -

A) UNIAXIAL* SYNOVIAL JOINTS Type
Color Code Important JOINTS Doctors Notes Editing file Notes/Extra explanation Objectives By the end of the lecture, students should be able to: Define the term “Joint” . Describe the classification of the 3 types of joints & give an example of each. Describe the characteristics of synovial joints. Describe the classification of synovial joints & give an example of each. List factors maintaining stability of joints. Recite “Hilton’s law” for nerve supply of joints. Definition What is a joint? It is the site where two or more bones meet together. Another def. : it is the meeting of two or more articulating joints Classification of joints Joints are classified according to the tissues that lie between the bones into: A. Fibrous B. Cartilaginous C. Synovial 1) Fibrous Joints The articulating surfaces are joined by fibrous connective tissue, where No or very mild movement. 1. Skull sutures: Temporary (as it ossify later) 2. Inferior tibiofibular joints (syndesmosis): minimal movement, permanent joints. 3. Gomphosis: dental alveolar joints. Articulation between root of the tooth with mandible or maxilla مهمة اﻷمثلة تجي عليها أسئلة )المثال وﻷي نوع( • 2) Cartilaginous Joints When two bones are joined by cartilage the formed joint is called “cartilaginous joint” and are classified into 2 types: o Primary Cartilaginous (synchondrosis): o The bones are united by a plate or a bar of hyaline cartilage. o No movement, temporary joints (ossify later). o Example: plate of hyaline cartilage o Between the Epiphysis and the Diaphysis of a growing bone. hyaline cartilage. o Between the First Rib and the Sternum (1st sternocostal joint). Any articulating surface will has a plate of hyaline cartilage plate of fibrocartilage (The rest of the sternocostal joints are synovial plane joints.) o Secondary Cartilaginous: o The bones are united by a plate of fibrocartilage. -

Chapter 7 Body Systems
Identification and Palpation of Individual Joints of the Body 1 Joints of the Skull Cranial sutures 2 The four cranial sutures are the coronal suture, the sagittal suture, the squamous suture, and the lambdoidal suture. The coronal suture is located on the top of the skull above the eyebrows. The sagittal suture is midway between the top of the ear and the eye. The squamous suture arcs above the ear, and the lambdoidal suture is behind the ear, just above the mastoid process in an arc superiorly and posteriorly. Joints of the Skull Temporomandibular joint 4 The temporomandibular joint consists of the temporal bone and mandible and is a synovial modified hinge joint. The TMJ is one of the strongest joints in the body and is the only biarticular joint in the body. Joints of the Shoulder Glenohumeral joint 6 The glenohumeral joint is the main joint in the shoulder and the most mobile joint in the human body. The previous figure shows the ligaments of the shoulder. Joints of the Shoulder Sternoclavicular joint 8 The movements of the sternoclavicular joint follow the movements of the scapula and clavicle because no muscle works directly on this joint. A decrease or loss of mobility in this joint directly affects shoulder movement. This joint is the only direct connection between the axial skeleton and the shoulder girdle and arm. Joints of the Shoulder Acromioclavicular 10 Although a small joint, the acromioclavicular joint is important for shoulder movements. Some people do not have an acromioclavicular joint because the bones have fused. Joints of the Elbow Ulnohumeral and radiohumeral joints 12 These two joints allow flexion and extension. -

Student Workbook Answer Pages Italicized Page Numbers After the Answers Indicate Where the Informa- Matching 5) Deep Fascia Tion Can Be Found in Trail Guide
Student Workbook Answer Pages Italicized page numbers after the answers indicate where the informa- Matching 5) deep fascia tion can be found in Trail Guide. 1) N adipose—p. 17 6) adipose (fatty) tissue 2) F aponeurosis—p. 13 7) superficial fascia 3) D artery—p. 16 8) skin 4) H bone—p. 10 9) deep fascia Introduction 5) E bursa—p. 16 Tour Guide Tips #1, p. 1 6) B fascia—p. 14 1) bony landmarks—p. 2 7) G ligament—p. 13 2) Even though the topography, 8) I lymph node—p. 17 Navigating shape and proportion are unique, 9) A muscle—p. 11 Regions of the Body, p. 6 the body’s composition and struc- 10) J nerve—p. 17 1) pectoral tures are virtually identical on all 11) K retinaculum—p. 15 2) axillary individuals.—p. 2 12) L skin—p. 10 3) brachial 3) To examine or explore by touch- 13) M tendon—p. 13 4) cubital ing (an organ or area of the body), 14) C vein—p. 16 5) abdominal usually as a diagnostic aid—p. 4 6) inguinal 4) locating, aware, assessing—p. 4 Exploring Textures #1, p. 3 7) pubic 5) directs movement, depth.—p. 4 1) epidermis 8) femoral 6) • read the information 2) dermis 9) facial • visualize what you are trying 3) arrector pili muscle 10) mandibular to access 4) sweat gland 11) supraclavicular • verbalize to your partner what 5) hair follicle 12) antecubital you feel 6) blood vessels 13) patellar • locate the structure first 7) muscle fibers 14) crural on yourself 8) endomysium 15) cranial • read the text aloud 9) perimysium 16) cervical • be patient—p. -

Powerpoint Handout: Lab 1, Thorax
PowerPoint Handout: Lab 1, Thorax Slide Title Slide Number Slide Title Slide Number Thorax & Thoracic Cavity: Introduction Slide 2 Visceral Pleura Slide 21 Thoracic Cavity Apertures Slide 3 Pneumothorax, Pleural Effusion, Hemothorax Slide 22 Osseous Thorax: Sternum Slide 4 Types of Pneumothorax Slide 23 Osseous Thorax: Sternal Angle and Transverse Thoracic Plane Slide 5 Pleural Recesses Slide 24 Osseous Thorax: Ribs Slide 6 Costodiaphragmatic Recess and Costophrenic Angle Slide 25 Osseous Thorax: Ribs Slide 7 Pleurisy and Referred Pain Slide 26 Osseous Thorax: Ribs Slide 8 Diaphragm Introduction Slide 27 Supernumerary Ribs Slide 9 Diaphragm Apertures Slide 28 Osseous Thorax: Rib Joints Slide 10 Diaphragm Motor Innervation Slide 29 Muscular Thorax: Intercostal Muscles Slide 11 Diaphragm Movements Slide 30 Muscular Thorax: Intercostal Muscles (Continued) Slide 12 Diaphragm Sensory Innervation Slide 31 Intercostal Spaces and Intercostal Neurovascular Bundles Slide 13 Lung Surfaces Slide 32 Intercostal Neurovascular Bundle Slide 14 Root of the Lung Slide 33 Intercostal Nerve Block Slide 15 Slide 34 Internal Thoracic (Mammary) Artery Slide 16 Lung Lobes and Fissures Summary of of Intercostal Vasculature Slide 17 Contact Impressions on Mediastinal Lung Surface (Right) Slide 35 Collateral Circulation Through Internal Thoracic Artery Slide 18 Contact Impressions on Mediastinal Lung Surface (Left) Slide 36 Thoracic Cavity Subdivisions Slide 19 Surface Anatomy Correlates of Lung Lobes and Fissures Slide 37 Pleura and Endothoracic Fascia Slide 20 Lung Auscultation Slide 38 Thorax & Thoracic Cavity: Introduction The thorax refers to the region of the body between the neck https://3d4medic.al/enFsQOFf and the abdomen. The thoracic cavity is an irregularly shaped cylinder enclosed by the musculoskeletal walls of the thorax and the diaphragm. -

Alligator Mississippiensis): an XROMM Analysis Robert J
© 2017. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2017) 220, 3181-3190 doi:10.1242/jeb.156166 RESEARCH ARTICLE Rib kinematics during lung ventilation in the American alligator (Alligator mississippiensis): an XROMM analysis Robert J. Brocklehurst1,*, Sabine Moritz2, Jonathan Codd3, William I. Sellers1 and Elizabeth L. Brainerd2 ABSTRACT these, the two most important are costal aspiration and the hepatic The current hypothesis regarding the mechanics of breathing in piston (Movie 1) (Claessens, 2009b). crocodylians is that the double-headed ribs, with both a capitulum and The mechanics of the hepatic piston are relatively straightforward tuberculum, rotate about a constrained axis passing through the two and have been extensively studied (Farmer and Carrier, 2000; articulations; moreover, this axis shifts in the caudal thoracic ribs, as Uriona and Farmer, 2006; Munns et al., 2012), but costal aspiration the vertebral parapophysis moves from the centrum to the transverse is more complex. Ventilation is powered by the hypaxial muscles, process. Additionally, the ventral ribcage in crocodylians is thought to with the transverse abdominal responsible for exhalation, and the possess additional degrees of freedom through mobile intermediate intercostal muscles powering inhalation and exhalation (Gans and ribs. In this study, X-ray reconstruction of moving morphology Clark, 1976; Carrier, 1989; Brainerd and Owerkowicz, 2006). (XROMM) was used to quantify rib rotation during breathing in However, muscles can only do positive work and hence contribute American alligators. Whilst costovertebral joint anatomy predicted to ventilation when actively shortening, but in order to power overall patterns of motion across the ribcage (decreased bucket inhalation this muscular contraction must be converted into handle motion and increased calliper motion), there were significant expansion of the thorax (Brainerd and Owerkowicz, 2006; deviations: anatomical axes overestimated pump handle motion and, Brainerd, 2015). -

The Finite Element Analysis of the Human Rib Cage
International Journal of Modern Engineering Research (IJMER) www.ijmer.com Vol.2, Issue.2, Mar-Apr 2012 pp-046-049 ISSN: 2249-6645 THE FINITE ELEMENT ANALYSIS OF THE HUMAN RIB CAGE ROSHAN P. GHODKHANDE. Prof .AJINKYA P. EDLABADKAR Department Of Mechanical Engg. Department Of Mechanical Engg. Yeshwantrao Chavan College Of Engineering Nagpur, Yeshwantrao Chavan College Of Engineering Nagpur, Maharashtra, India Maharashtra, India. Abstract In this paper, finite element analysis of the rib cage model is latter are attached to thoracic vertebrae (see Fig. 1). The ribs applied to, recognize stress distributions and to determine and the sternum contain red bone marrow capable of the rate of bone fractures (especially for pathologically hematopoiesis. changed bones). Also to determine the load and stress to occurs on the human rib cage at any accident. Key words: finite element model, thorax, rib cage, Nuss implant, pectus Excavatum, fail chest. Introduction Generally, frontal impacts are considered to be the most common vehicle colli- sions causing injuries. This paper describes development and validation of a thorax finite element model of a 15-25 years old child. The thorax model is developed in order to perform more detailed investigation of the human rib cage responses and injuries subject to impact loads. Antropho- metric data of thorax is obtained from measurements and from drawings of crossections found in atlases of the human anatomy. Let us begin first from a brief description of the rib cage anatomy. The skeleton of thorax or chest is an Osseo-cartilaginous cage Fig. 1. Thorax anatomy containing and pro- tecting principal organs of respiration and circulation. -

General Arthrology (Joints)
General arthrology (Joints) 2 types of joints : 1. Synarthrosis 2. Diarthrosis 1. Synarthrosis (connective tissue, cartilage, bone) -connection by some kind of connective tissue : fibrous tissue, cartilage or bone A. fibrous joint (articulatio fibrosa, syndesmosis) - (sutures, ligaments, Gomphosis) a) 3 types of sutures sutura serrata - sutura squamosa – squamous suture sutura plana – flat suture b) membrana interossea antebrachii, various ligaments c) gomphosis (socket) – dentoalveolar syndesmosis B. cartilaginous joint (articulatio cartilaginea, synchondrosis) – bones are linked by cartilage, nearly immobile continuous connection of bones C. synostosis (by bone tissue, origin of the bones was isolated) 2. Diarthrosis (articulatio synovialis) - Synovial joint Movable connection of 2 or more bones by touch or contact Art. surfaces covered by articular cartilage General features of a joint. Facies articulares- articular surfaces Caput articulare – articular head Fossa (fovea) articularis – articular fossa Cartilago articularis – articular cartilage Capsula articularis – articular capsule (joint capsule) Membrana fibrosa, stratum fibrosum– fibrous membrane, layer Membrana synovialis, stratum synoviale – synovial membrane, inner layer Plicae synoviales Villi synoviales Synovia – synovial fluid Rete articulare – articular network of arteries Cavitas articularis – articular cavity, joint cavity Additional joint structures, features of the joint Labrum articulare - Disci et menisci – articular discs and meniscs (difference) Ligamenta - ligaments -

Spinal Mobility with Concept of the Combination of Motion Spheres In
https://doi.org/10.33805/2641-8991.122 Volume 2 Issue 1 | PDF 122 | Pages 2 Neurophysiology and Rehabilitation Commentary ISSN: 2641-8991 Spinal Mobility with Concept of the Combination of Motion Spheres in the Body *1,2 3 4,5 Hiroshi Bando , Mitsuru Murakami and Akito Moriyasu Affiliation 1Japan Masters Athletics, Tokushima division, board, Tokushima, Japan 2Tokushima University/Medical Research, Tokushima, Japan 3 Japan Masters Athletics, Kagawa division, vice-president, Kagawa, Japan 4Fellow of Spine Dynamic Therapy Association (SDTA), Japan 5Fellow of the Japan Athletes Rehabilitation Trainers Association (JARTA), Japan *Corresponding author: Hiroshi Bando, Tokushima University/Medical Research, Nakashowa 1-61, Tokushima 770-0943, Japan, Tel: +81-90-3187-2485, Email: [email protected] Citation: Bando H, Murakami M and Moriyasu A. Spinal mobility with concept of the combination of motion spheres in the body (2019) Neurophysio and Rehab 2: 51-52. Received: Sep 18, 2019 Accepted: Sep 25, 2019 Published: Oct 03, 2019 Copyright: © 2019 Bando H, et al., This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Keywords: Spinal mobility, Motion sphere, Range of Motion (ROM), Sagittal plane, Sternocostal joints and Sacroiliac joints. Abbreviations: ROM-Range of Motion. In recent years, the importance of physical rehabilitation has been more one, distinguishing between the upper and lower body [8,9]. Among emphasized. The authors and colleagues have continued practice and the operations of three movements mentioned above, twisting is the research for long [1,2]. -

Contribution to 3D Modelling of the Human Thorax in Breathing Movement : in Vivo Analysis of Thorax Joint Kinematics Benoit Beyer
Contribution to 3D modelling of the human thorax in breathing movement : in vivo analysis of thorax joint kinematics Benoit Beyer To cite this version: Benoit Beyer. Contribution to 3D modelling of the human thorax in breathing movement : in vivo analysis of thorax joint kinematics. Biomechanics [physics.med-ph]. Université de Lyon, 2016. English. NNT : 2016LYSE1309. tel-01458941v3 HAL Id: tel-01458941 https://tel.archives-ouvertes.fr/tel-01458941v3 Submitted on 31 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Contribution à la modélisation 3D du thorax humain durant le mouvement respiratoire: Analyse in vivo de la cinématique des articulations du thorax UNIVERSITÉ LIBRE DE BRUXELLES Faculté de Médecine : Laboratoire d’Anatomie, Biomécanique et Organogenèse (LABO) Faculté des Sciences de la Motricité : Laboratoire d’Anatomie Fonctionnelle (LAF) Brussels, Belgium UNIVERSITÉ CLAUDE BERNARD LYON1 Laboratoire de Biomécanique et Mécanique des Chocs (LBMC), Ecole doctorale MEGA, UMR_T9406, F69622 Lyon, France Benoit BEYER Doctoral thesis in Biomedical and Pharmaceutical Sciences, Brussels, Belgium, 2016 Doctoral thesis in Biomechanics, Lyon, France, 2016 Promoters: Pr. Serge VAN SINT JAN Université Libre de Bruxelles, Laboratory of Anatomy, Biomechanics and Organogenesis (L.A.B.O), Faculty of Medicine (ULB, Brussels, Belgium) Pr. -

Temporomandibular Joint
ARTICULATIONS OF THE SPINE AND THORAX Pages 8 -12, 42 and 57 Arthrology . joint, articulation or union between two or more bones . Classification by degree of movement or tissue that bind the bones together By Degree of Movement . synarthrodial joint - allows no movement; flat bones of the skull . amphiarthrodial joint - partially movable . diarthrodial joint - freely movable By Joint Tissues . fibrous connective tissue . cartilage . combination of connective tissue and cartilage . cartilage and joint cavity The Fibrous Joint . suture - between bones of the skull . syndesmosis - partially movable; two bones connected by a fibrous interosseous membrane . gomphosis - articulation between the teeth and the alveolar processes The Synchondroses . primary cartilaginous joint . plate of hyaline cartilage between apposing surfaces = area of growth between bones . sphenooccipital synchondrosis in the young → fuses after adolescence The Symphyses . secondary cartilaginous joint . partially movable . apposing bony surfaces are covered with cartilage but separated by intervening fibrous tissue or fibrocartilage . intervertebral discs, symphysis pubis The Synovial Joint . freely movable . surfaces of the opposing bones are covered by articular cartil. the inner aspect of articular cavity is lined with a synovial membrane (not articular surfaces of the cartilage) → produces intervening film of synovial fluid . some joints contain discs or meniscus interposed between articular surfaces . reinforcing ligaments . bursae - flattened sacs that contain synovial fluid and reduce friction . tendon sheath - bursa that wraps around a tendon that is subject to friction The Multiaxial Joint . provides the greatest degree of movement in three planes . Ball-and-Socket and Saddle/Ellipsoid joint The Biaxial Joint . allows movement in two planes . the shape of the joint surfaces prevents rotation around a vertical axis .