Alligator Mississippiensis): an XROMM Analysis Robert J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anklyosed Spine: Fractures in DISH and Anklyosing Spondylitis
The Anklyosed Spine: Fractures in DISH and Anklyosing Spondylitis Lee F. Rogers, MD ACCR, Oct 26, 2012 Dallas, Texas Diffuse idiopathic skeletal hyperostosis (DISH) and ankylosing spondylitis (AS) are the most common diseases associated with spinal ankylosis. While they share the characteristic of ankylosis they are, in fact two separate diseases with distinct clinical, pathologic, and radiologic features. DISH: Diffuse idiopathic skeletal hyperostosis is a disease of older persons characterized by extensive ossification of the paraspinal ligaments anteriorly and laterally, bridging the intervening disc spaces. Bony bridging may be continuous or discontinuous. The anterior cortex of the vertebral body can be seen within the ossification. These findings are much more pronounced in the thoracic and lower cervical spine than in the lumbar area. Minor expressions of this disorder are commonly encountered in the mid-dorsal spine on lateral views of the chest. DISH characteristically spares the SI joints. The SI joints remain patent and clearly visible on radiographs or CT even in the presence of extensive disease in the thoracolumbar and cervical spine. DISH is also characterized by enthesopathy; ossification of ligaments or tendon insertions, forming so-called entheses. These appear as whiskering of the iliac crest, ischial tuberosites and greater trochanters. The radiographic appearance bears a superficial resemblance to that of AS. In DISH the spinal ossification is very irregular and unlike the thin, vertical syndesmophytes see in AS. The relative absence of changes in the lumbosacral spine, the patency of the SI joints, and absence of ankylosis of the facet and costovertebral joints should allow differentiation of DISH from AS. -

Diapositiva 1
Thoracic Cage and Thoracic Inlet Professor Dr. Mario Edgar Fernández. Parts of the body The Thorax Is the part of the trunk betwen the neck and abdomen. Commonly the term chest is used as a synonym for thorax, but it is incorrect. Consisting of the thoracic cavity, its contents, and the wall that surrounds it. The thoracic cavity is divided into 3 compartments: The central mediastinus. And the right and left pulmonary cavities. Thoracic Cage The thoracic skeleton forms the osteocartilaginous thoracic cage. Anterior view. Thoracic Cage Posterior view. Summary: 1. Bones of thoracic cage: (thoracic vertebrae, ribs, and sternum). 2. Joints of thoracic cage: (intervertebral joints, costovertebral joints, and sternocostal joints) 3. Movements of thoracic wall. 4. Thoracic cage. Thoracic apertures: (superior thoracic aperture or thoracic inlet, and inferior thoracic aperture). Goals of the classes Identify and describe the bones of the thoracic cage. Identify and describe the joints of thoracic cage. Describe de thoracic cage. Describe the thoracic inlet and identify the structures passing through. Vertebral Column or Spine 7 cervical. 12 thoracic. 5 lumbar. 5 sacral 3-4 coccygeal Vertebrae That bones are irregular, 33 in number, and received the names acording to the position which they occupy. The vertebrae in the upper 3 regions of spine are separate throughout the whole of life, but in sacral anda coccygeal regions are in the adult firmly united in 2 differents bones: sacrum and coccyx. Thoracic vertebrae Each vertebrae consist of 2 essential parts: An anterior solid segment: vertebral body. The arch is posterior an formed of 2 pedicles, 2 laminae supporting 7 processes, and surrounding a vertebral foramen. -

Ligaments of the Costovertebral Joints Including Biomechanics, Innervations, and Clinical Applications: a Comprehensive Review W
Open Access Review Article DOI: 10.7759/cureus.874 Ligaments of the Costovertebral Joints including Biomechanics, Innervations, and Clinical Applications: A Comprehensive Review with Application to Approaches to the Thoracic Spine Erfanul Saker 1 , Rachel A. Graham 2 , Renee Nicholas 3 , Anthony V. D’Antoni 2 , Marios Loukas 1 , Rod J. Oskouian 4 , R. Shane Tubbs 5 1. Department of Anatomical Sciences, St. George's University School of Medicine, Grenada, West Indies 2. Department of Anatomy, The Sophie Davis School of Biomedical Education 3. Department of Physical Therapy, Samford University 4. Neurosurgery, Complex Spine, Swedish Neuroscience Institute 5. Neurosurgery, Seattle Science Foundation Corresponding author: Erfanul Saker, [email protected] Abstract Few studies have examined the costovertebral joint and its ligaments in detail. Therefore, the following review was performed to better elucidate their anatomy, function and involvement in pathology. Standard search engines were used to find studies concerning the costovertebral joints and ligaments. These often- overlooked ligaments of the body serve important functions in maintaining appropriate alignment between the ribs and spine. With an increasing interest in minimally invasive approaches to the thoracic spine and an improved understanding of the function and innervation of these ligaments, surgeons and clinicians should have a good working knowledge of these structures. Categories: Neurosurgery, Orthopedics, Rheumatology Keywords: costovertebral joint, spine, anatomy, thoracic Introduction And Background The costovertebral joint ligaments are relatively unknown and frequently overlooked anatomical structures [1]. Although small and short in size, they are abundant, comprising 108 costovertebral ligaments in the normal human thoracic spine, and they are essential to its stability and function [2-3]. -

Chapter 21 Fractures of the Upper Thoracic Spine: Approaches and Surgical Management
Chapter 21 Fractures of the Upper Thoracic Spine: Approaches and Surgical Management Sean D Christie, M.D., John Song, M.D., and Richard G Fessler, M.D., Ph.D. INTRODUCTION Fractures occurring in the thoracic region account for approximately 17 to 23% of all traumatic spinal fractures (1), with 22% of traumatic spinal fractures occurring between T1 and T4 (16). More than half of these fractures result in neurological injury, and almost three-quarters of those impaired suffer from complete paralysis. Obtaining surgical access to the anterior vertebral elements of the upper thoracic vertebrae (T1–T6) presents a unique anatomic challenge. The thoracic cage, which narrows significantly as it approaches the thoracic inlet, has an intimate association between the vertebral column and the superior mediastinal structures. The supraclavicular, transmanubrial, transthoracic, and lateral parascapular extrapleural approaches each provide access to the anterior vertebral elements of the upper thoracic vertebrae. However, each of these approaches has distinct advantages and disadvantages and their use should be tailored to each individual patient’s situation. This chapter reviews these surgical approaches. Traditional posterior approaches are illustrated in Figure 21.1, but will not be discussed in depth here. ANATOMIC CONSIDERATIONS AND STABILITY The upper thoracic spine possesses unique anatomic and biomechanical properties. The anterior aspects of the vertebral bodies are smaller than the posterior aspects, which contribute to the physiological kyphosis present in this region of the spine. Furthermore, this orientation results in a ventrally positioned axis of rotation, predisposing this region to compression injuries. The combination and interaction of the vertebral bodies, ribs, and sternum increase the inherent biomechanical stability of this segment of the spine to 2 to 3 times that of the thoracolumbar junction. -

Canine Thoracic Costovertebral and Costotransverse Joints Three Case Reports of Dysfunction and Manual Therapy Guidelines for A
Topics in Compan An Med 29 (2014) 1–5 Topical review Canine Thoracic Costovertebral and Costotransverse Joints: Three Case Reports of Dysfunction and Manual Therapy Guidelines for Assessment and Treatment of These Structures Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRTn Keywords: The costovertebral and costotransverse joints receive little attention in research. However, pain costovertebral associated with rib articulation dysfunction is reported to occur in human patients. The anatomic costotransverse structures of the canine rib joints and thoracic spine are similar to those of humans. As such, it is ribs physical therapy proposed that extrapolation from human physical therapy practice could be used for the assessment and rehabilitation treatment of the canine patient with presumed rib joint pain. This article presents 3 case studies that manual therapy demonstrate signs of rib dysfunction and successful treatment using primarily physical therapy manual techniques. General assessment and select treatment techniques are described. & 2014 Elsevier Inc. All rights reserved. The Canine Fitness Centre Ltd, Calgary, Alberta, Canada nAddress reprint requests to Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRT, The Canine Fitness Centre Ltd, 509—42nd Ave SE, Calgary, Alberta, Canada T2G 1Y7 E-mail: [email protected] The articular structures of the thorax comprise facet joints, the erect spine and further presented that in reviewing the literature, intervertebral disc, and costal joints. Little research has been they were unable to find mention of natural development of conducted on these joints in human or animal medicine. However, idiopathic scoliosis in quadrupeds; however, there are reports of clinical case presentations in human journals, manual therapy avian models and adolescent models in man. -

1 the Thoracic Wall I
AAA_C01 12/13/05 10:29 Page 8 1 The thoracic wall I Thoracic outlet (inlet) First rib Clavicle Suprasternal notch Manubrium 5 Third rib 1 2 Body of sternum Intercostal 4 space Xiphisternum Scalenus anterior Brachial Cervical Costal cartilage plexus rib Costal margin 3 Subclavian 1 Costochondral joint Floating ribs artery 2 Sternocostal joint Fig.1.3 3 Interchondral joint Bilateral cervical ribs. 4 Xiphisternal joint 5 Manubriosternal joint On the right side the brachial plexus (angle of Louis) is shown arching over the rib and stretching its lowest trunk Fig.1.1 The thoracic cage. The outlet (inlet) of the thorax is outlined Transverse process with facet for rib tubercle Demifacet for head of rib Head Neck Costovertebral T5 joint T6 Facet for Tubercle vertebral body Costotransverse joint Sternocostal joint Shaft 6th Angle rib Costochondral Subcostal groove joint Fig.1.2 Fig.1.4 A typical rib Joints of the thoracic cage 8 The thorax The thoracic wall I AAA_C01 12/13/05 10:29 Page 9 The thoracic cage Costal cartilages The thoracic cage is formed by the sternum and costal cartilages These are bars of hyaline cartilage which connect the upper in front, the vertebral column behind and the ribs and intercostal seven ribs directly to the sternum and the 8th, 9th and 10th ribs spaces laterally. to the cartilage immediately above. It is separated from the abdominal cavity by the diaphragm and communicates superiorly with the root of the neck through Joints of the thoracic cage (Figs 1.1 and 1.4) the thoracic inlet (Fig. -

Background and Anatomy
BACKGROUND AND ANATOMY CASE REPORT BACKGROUND Painful musculoskeletal disorders in the region of the thoracic spine can be difficult to diagnose and treat. Imaging examinations are often negative or difficult to visualize, especially in instances of internal disc disruption, costovertebral arthropathies, and zygapophyseal joint problems.16,23,48,53,71 Knowledge of some of the distinctive anatomical features of this region, combined with information gathered in biomechanical studies2,6,11,15,35,43,44,55,61,62 can assist the clinician in isolating a painful structure (or structures) based on clinical examination. Because spinal metastases are most often seen in the thoracic spine,73,37 and because organic problems can cause musculoskeletal-like pain, it is important that the patient first undergo a thorough medical work up prior to visiting the physical therapist. When dealing with painful musculoskeletal conditions of the thoracic spine, there are a number of unique features to consider about this region. Anatomical construction of the thoracic spine makes it a rigid structure and, enhanced by the rib cage, it functions not only to protect the life-sustaining organs in the thorax, but it also serves as the foundation for movements of the head and extremities. The presence of the rib cage alone adds a number of possible sources of pain, such as pathology at the costovertebral (dorsal) and even cost-chondro-sternal (ventral) connections. The thoracic spine has the largest number of vertebrae of the three spinal regions. Morphological variation between the cranial and the caudal vertebrae is great, and this difference in morphology illustrates one of the primary functions of the thoracic spine, which is that of a transition “zone.” The cranial segments, (T1 to T4) and the caudal segments, (T8 to T12) have the appearance and the kinematic features similar to that of the cervical and lumbar spines, respectively. -
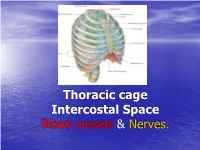
Thorax Is Upper Part of Trunk •Study of Thorax by Studying: –Wall of the Thorax –Contents of Thorax Wall of Thorax
Thoracic cage Intercostal Space Blood vessels & Nerves. General Overview •Thorax is upper part of trunk •Study of thorax by studying: –Wall of the thorax –Contents of thorax Wall of thorax •Skeletal framework-Thoracic cage •Muscles, membrane & ligaments Thoracic cage Thoracic Cage • Conical in shape with truncated apex • Boundaries: • Anteriorly : Sternum • Posteriorly: Twelve Thoracic Vertebrae • On sides : Twelve pairs of Ribs & Costal Cartilages Thoracic cage • The thoracic wall is covered from outside to inside by • skin • superficial facia • deep fascia & • extrinsic muscle. • Muscles of the upper limb: • Pectoralis major Pectoralis minor • Trapezius, Serratus anterior, lattisimus dorsi • Levator scapulae, Rhomboideus major & minor • Serratus posterior superior & Inferior. • Muscles of the abdomen: • Rectus abdominis & external oblique • Muscle of the back: Erector spinae Ribs • True Ribs: articulating with sternum anteriorly – Vertebrosternal ribs: 1-7 ribs • False Ribs: Do not articulate with sternum anteriorly – Vertebrochondral ribs: 8-10 ribs – Floating ribs: 11-12 ribs Ribs First & 12th ribs Apertures of thorax • Superior Aperture (Inlet of Thorax) communicates thorax & neck. Structures passing between neck & thorax are contents of inlet. Partly covered by suprapleural membrane. • Inferior Aperture: Closed by Diaphragm. Structures passing between thorax & Abdomen pass through openings in diaphragm Joints of thorax Joints Joints Joints of thorax • Intervertebral joints: joints between bodies of vertebrae. It is Symphyseal type of joint. • Joint between articular processes of vertebrae is Plane synovial joint • Costovertebral joint: It is plane synovial joint between rib & vertebrae. • Manubriosternal joint: Between manubrium & Body of sternum, Symphyseal joint. • Xiphisternal joint: Between body of sternum & xiphoid process, symphyseal joint Joints of thorax • Sternocostal joints: – First Sternocostal joint between first rib & sternum is primary cartilaginous joint which is responsible for movement of sternum during respiration. -

A) UNIAXIAL* SYNOVIAL JOINTS Type
Color Code Important JOINTS Doctors Notes Editing file Notes/Extra explanation Objectives By the end of the lecture, students should be able to: Define the term “Joint” . Describe the classification of the 3 types of joints & give an example of each. Describe the characteristics of synovial joints. Describe the classification of synovial joints & give an example of each. List factors maintaining stability of joints. Recite “Hilton’s law” for nerve supply of joints. Definition What is a joint? It is the site where two or more bones meet together. Another def. : it is the meeting of two or more articulating joints Classification of joints Joints are classified according to the tissues that lie between the bones into: A. Fibrous B. Cartilaginous C. Synovial 1) Fibrous Joints The articulating surfaces are joined by fibrous connective tissue, where No or very mild movement. 1. Skull sutures: Temporary (as it ossify later) 2. Inferior tibiofibular joints (syndesmosis): minimal movement, permanent joints. 3. Gomphosis: dental alveolar joints. Articulation between root of the tooth with mandible or maxilla مهمة اﻷمثلة تجي عليها أسئلة )المثال وﻷي نوع( • 2) Cartilaginous Joints When two bones are joined by cartilage the formed joint is called “cartilaginous joint” and are classified into 2 types: o Primary Cartilaginous (synchondrosis): o The bones are united by a plate or a bar of hyaline cartilage. o No movement, temporary joints (ossify later). o Example: plate of hyaline cartilage o Between the Epiphysis and the Diaphysis of a growing bone. hyaline cartilage. o Between the First Rib and the Sternum (1st sternocostal joint). Any articulating surface will has a plate of hyaline cartilage plate of fibrocartilage (The rest of the sternocostal joints are synovial plane joints.) o Secondary Cartilaginous: o The bones are united by a plate of fibrocartilage. -

Biomechanics of Thoracic Discectomy
Neurosurg Focus 11 (3):Article 6, 2001, Click here to return to Table of Contents Biomechanics of thoracic discectomy ANDREW E. WAKEFIELD, M.D., MICHAEL P. STEINMETZ, M.D., AND EDWARD C. BENZEL, M.D. Department of Neurosurgery, Hartford Hospital, Hartford, Connecticut; and The Cleveland Clinic Foundation, Department of Neurosurgery, Cleveland, Ohio The thoracic spine is a structurally unique region that renders it uniquely suceptible to thoracic disc herniation. Surgical management strategies are complicated, in part, by the regional anatomical and biomechanical nuances. Surgical approaches include posterior, posterolateral, and anterior routes. Each isassociated with specific indications and contraindications. The biomechanical principles and safe anatomical trajectories must be considered in the surgical deci- sion-making process. These issues are discussed in the pages that follow. KEY WORDS • biomechanics • thoracic spine • instability • discectomy The thoracic spine has structurally distinct characteris- RELEVANT THORACIC ANATOMY tics that distinguish it from the cervical and lumbar spine. The posterior thoracic VB height is greater than the an- A highly specialized region, it is uniquely adapted to an terior height, which explains, in part, the existence of tho- erect posture and the load-bearing demands required from 5 racic kyphosis. The size of the thoracic VBs increases as the daily activities of upright creatures. The thoracic the thoracic spine descends. The facet joints change their spine has been divided into three regions: 1) the cervical- orientation from the coronal plane in the cervicothoracic to-thoracic transition zone (T1–4) 2), the thoracic-to-lum- region to a more sagittal plane in the thoracolumbar re- bar transition zone (T10–12), and 3) the midthoracic re- 3 16 gion, and this affects spinal motion. -

Chapter 7 Body Systems
Identification and Palpation of Individual Joints of the Body 1 Joints of the Skull Cranial sutures 2 The four cranial sutures are the coronal suture, the sagittal suture, the squamous suture, and the lambdoidal suture. The coronal suture is located on the top of the skull above the eyebrows. The sagittal suture is midway between the top of the ear and the eye. The squamous suture arcs above the ear, and the lambdoidal suture is behind the ear, just above the mastoid process in an arc superiorly and posteriorly. Joints of the Skull Temporomandibular joint 4 The temporomandibular joint consists of the temporal bone and mandible and is a synovial modified hinge joint. The TMJ is one of the strongest joints in the body and is the only biarticular joint in the body. Joints of the Shoulder Glenohumeral joint 6 The glenohumeral joint is the main joint in the shoulder and the most mobile joint in the human body. The previous figure shows the ligaments of the shoulder. Joints of the Shoulder Sternoclavicular joint 8 The movements of the sternoclavicular joint follow the movements of the scapula and clavicle because no muscle works directly on this joint. A decrease or loss of mobility in this joint directly affects shoulder movement. This joint is the only direct connection between the axial skeleton and the shoulder girdle and arm. Joints of the Shoulder Acromioclavicular 10 Although a small joint, the acromioclavicular joint is important for shoulder movements. Some people do not have an acromioclavicular joint because the bones have fused. Joints of the Elbow Ulnohumeral and radiohumeral joints 12 These two joints allow flexion and extension. -

Student Workbook Answer Pages Italicized Page Numbers After the Answers Indicate Where the Informa- Matching 5) Deep Fascia Tion Can Be Found in Trail Guide
Student Workbook Answer Pages Italicized page numbers after the answers indicate where the informa- Matching 5) deep fascia tion can be found in Trail Guide. 1) N adipose—p. 17 6) adipose (fatty) tissue 2) F aponeurosis—p. 13 7) superficial fascia 3) D artery—p. 16 8) skin 4) H bone—p. 10 9) deep fascia Introduction 5) E bursa—p. 16 Tour Guide Tips #1, p. 1 6) B fascia—p. 14 1) bony landmarks—p. 2 7) G ligament—p. 13 2) Even though the topography, 8) I lymph node—p. 17 Navigating shape and proportion are unique, 9) A muscle—p. 11 Regions of the Body, p. 6 the body’s composition and struc- 10) J nerve—p. 17 1) pectoral tures are virtually identical on all 11) K retinaculum—p. 15 2) axillary individuals.—p. 2 12) L skin—p. 10 3) brachial 3) To examine or explore by touch- 13) M tendon—p. 13 4) cubital ing (an organ or area of the body), 14) C vein—p. 16 5) abdominal usually as a diagnostic aid—p. 4 6) inguinal 4) locating, aware, assessing—p. 4 Exploring Textures #1, p. 3 7) pubic 5) directs movement, depth.—p. 4 1) epidermis 8) femoral 6) • read the information 2) dermis 9) facial • visualize what you are trying 3) arrector pili muscle 10) mandibular to access 4) sweat gland 11) supraclavicular • verbalize to your partner what 5) hair follicle 12) antecubital you feel 6) blood vessels 13) patellar • locate the structure first 7) muscle fibers 14) crural on yourself 8) endomysium 15) cranial • read the text aloud 9) perimysium 16) cervical • be patient—p.