Chapter 21 Fractures of the Upper Thoracic Spine: Approaches and Surgical Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anklyosed Spine: Fractures in DISH and Anklyosing Spondylitis
The Anklyosed Spine: Fractures in DISH and Anklyosing Spondylitis Lee F. Rogers, MD ACCR, Oct 26, 2012 Dallas, Texas Diffuse idiopathic skeletal hyperostosis (DISH) and ankylosing spondylitis (AS) are the most common diseases associated with spinal ankylosis. While they share the characteristic of ankylosis they are, in fact two separate diseases with distinct clinical, pathologic, and radiologic features. DISH: Diffuse idiopathic skeletal hyperostosis is a disease of older persons characterized by extensive ossification of the paraspinal ligaments anteriorly and laterally, bridging the intervening disc spaces. Bony bridging may be continuous or discontinuous. The anterior cortex of the vertebral body can be seen within the ossification. These findings are much more pronounced in the thoracic and lower cervical spine than in the lumbar area. Minor expressions of this disorder are commonly encountered in the mid-dorsal spine on lateral views of the chest. DISH characteristically spares the SI joints. The SI joints remain patent and clearly visible on radiographs or CT even in the presence of extensive disease in the thoracolumbar and cervical spine. DISH is also characterized by enthesopathy; ossification of ligaments or tendon insertions, forming so-called entheses. These appear as whiskering of the iliac crest, ischial tuberosites and greater trochanters. The radiographic appearance bears a superficial resemblance to that of AS. In DISH the spinal ossification is very irregular and unlike the thin, vertical syndesmophytes see in AS. The relative absence of changes in the lumbosacral spine, the patency of the SI joints, and absence of ankylosis of the facet and costovertebral joints should allow differentiation of DISH from AS. -

Diapositiva 1
Thoracic Cage and Thoracic Inlet Professor Dr. Mario Edgar Fernández. Parts of the body The Thorax Is the part of the trunk betwen the neck and abdomen. Commonly the term chest is used as a synonym for thorax, but it is incorrect. Consisting of the thoracic cavity, its contents, and the wall that surrounds it. The thoracic cavity is divided into 3 compartments: The central mediastinus. And the right and left pulmonary cavities. Thoracic Cage The thoracic skeleton forms the osteocartilaginous thoracic cage. Anterior view. Thoracic Cage Posterior view. Summary: 1. Bones of thoracic cage: (thoracic vertebrae, ribs, and sternum). 2. Joints of thoracic cage: (intervertebral joints, costovertebral joints, and sternocostal joints) 3. Movements of thoracic wall. 4. Thoracic cage. Thoracic apertures: (superior thoracic aperture or thoracic inlet, and inferior thoracic aperture). Goals of the classes Identify and describe the bones of the thoracic cage. Identify and describe the joints of thoracic cage. Describe de thoracic cage. Describe the thoracic inlet and identify the structures passing through. Vertebral Column or Spine 7 cervical. 12 thoracic. 5 lumbar. 5 sacral 3-4 coccygeal Vertebrae That bones are irregular, 33 in number, and received the names acording to the position which they occupy. The vertebrae in the upper 3 regions of spine are separate throughout the whole of life, but in sacral anda coccygeal regions are in the adult firmly united in 2 differents bones: sacrum and coccyx. Thoracic vertebrae Each vertebrae consist of 2 essential parts: An anterior solid segment: vertebral body. The arch is posterior an formed of 2 pedicles, 2 laminae supporting 7 processes, and surrounding a vertebral foramen. -
The Structure and Function of Breathing
CHAPTERCONTENTS The structure-function continuum 1 Multiple Influences: biomechanical, biochemical and psychological 1 The structure and Homeostasis and heterostasis 2 OBJECTIVE AND METHODS 4 function of breathing NORMAL BREATHING 5 Respiratory benefits 5 Leon Chaitow The upper airway 5 Dinah Bradley Thenose 5 The oropharynx 13 The larynx 13 Pathological states affecting the airways 13 Normal posture and other structural THE STRUCTURE-FUNCTION considerations 14 Further structural considerations 15 CONTINUUM Kapandji's model 16 Nowhere in the body is the axiom of structure Structural features of breathing 16 governing function more apparent than in its Lung volumes and capacities 19 relation to respiration. This is also a region in Fascla and resplrstory function 20 which prolonged modifications of function - Thoracic spine and ribs 21 Discs 22 such as the inappropriate breathing pattern dis- Structural features of the ribs 22 played during hyperventilation - inevitably intercostal musculature 23 induce structural changes, for example involving Structural features of the sternum 23 Posterior thorax 23 accessory breathing muscles as well as the tho- Palpation landmarks 23 racic articulations. Ultimately, the self-perpetuat- NEURAL REGULATION OF BREATHING 24 ing cycle of functional change creating structural Chemical control of breathing 25 modification leading to reinforced dysfunctional Voluntary control of breathing 25 tendencies can become complete, from The autonomic nervous system 26 whichever direction dysfunction arrives, for Sympathetic division 27 Parasympathetic division 27 example: structural adaptations can prevent NANC system 28 normal breathing function, and abnormal breath- THE MUSCLES OF RESPIRATION 30 ing function ensures continued structural adap- Additional soft tissue influences and tational stresses leading to decompensation. -

Vertebral Column and Thorax
Introduction to Human Osteology Chapter 4: Vertebral Column and Thorax Roberta Hall Kenneth Beals Holm Neumann Georg Neumann Gwyn Madden Revised in 1978, 1984, and 2008 The Vertebral Column and Thorax Sternum Manubrium – bone that is trapezoidal in shape, makes up the superior aspect of the sternum. Jugular notch – concave notches on either side of the superior aspect of the manubrium, for articulation with the clavicles. Corpus or body – flat, rectangular bone making up the major portion of the sternum. The lateral aspects contain the notches for the true ribs, called the costal notches. Xiphoid process – variably shaped bone found at the inferior aspect of the corpus. Process may fuse late in life to the corpus. Clavicle Sternal end – rounded end, articulates with manubrium. Acromial end – flat end, articulates with scapula. Conoid tuberosity – muscle attachment located on the inferior aspect of the shaft, pointing posteriorly. Ribs Scapulae Head Ventral surface Neck Dorsal surface Tubercle Spine Shaft Coracoid process Costal groove Acromion Glenoid fossa Axillary margin Medial angle Vertebral margin Manubrium. Left anterior aspect, right posterior aspect. Sternum and Xyphoid Process. Left anterior aspect, right posterior aspect. Clavicle. Left side. Top superior and bottom inferior. First Rib. Left superior and right inferior. Second Rib. Left inferior and right superior. Typical Rib. Left inferior and right superior. Eleventh Rib. Left posterior view and left superior view. Twelfth Rib. Top shows anterior view and bottom shows posterior view. Scapula. Left side. Top anterior and bottom posterior. Scapula. Top lateral and bottom superior. Clavicle Sternum Scapula Ribs Vertebrae Body - Development of the vertebrae can be used in aging of individuals. -

Ligaments of the Costovertebral Joints Including Biomechanics, Innervations, and Clinical Applications: a Comprehensive Review W
Open Access Review Article DOI: 10.7759/cureus.874 Ligaments of the Costovertebral Joints including Biomechanics, Innervations, and Clinical Applications: A Comprehensive Review with Application to Approaches to the Thoracic Spine Erfanul Saker 1 , Rachel A. Graham 2 , Renee Nicholas 3 , Anthony V. D’Antoni 2 , Marios Loukas 1 , Rod J. Oskouian 4 , R. Shane Tubbs 5 1. Department of Anatomical Sciences, St. George's University School of Medicine, Grenada, West Indies 2. Department of Anatomy, The Sophie Davis School of Biomedical Education 3. Department of Physical Therapy, Samford University 4. Neurosurgery, Complex Spine, Swedish Neuroscience Institute 5. Neurosurgery, Seattle Science Foundation Corresponding author: Erfanul Saker, [email protected] Abstract Few studies have examined the costovertebral joint and its ligaments in detail. Therefore, the following review was performed to better elucidate their anatomy, function and involvement in pathology. Standard search engines were used to find studies concerning the costovertebral joints and ligaments. These often- overlooked ligaments of the body serve important functions in maintaining appropriate alignment between the ribs and spine. With an increasing interest in minimally invasive approaches to the thoracic spine and an improved understanding of the function and innervation of these ligaments, surgeons and clinicians should have a good working knowledge of these structures. Categories: Neurosurgery, Orthopedics, Rheumatology Keywords: costovertebral joint, spine, anatomy, thoracic Introduction And Background The costovertebral joint ligaments are relatively unknown and frequently overlooked anatomical structures [1]. Although small and short in size, they are abundant, comprising 108 costovertebral ligaments in the normal human thoracic spine, and they are essential to its stability and function [2-3]. -
Part 1 the Thorax ECA1 7/18/06 6:30 PM Page 2 ECA1 7/18/06 6:30 PM Page 3
ECA1 7/18/06 6:30 PM Page 1 Part 1 The Thorax ECA1 7/18/06 6:30 PM Page 2 ECA1 7/18/06 6:30 PM Page 3 Surface anatomy and surface markings The experienced clinician spends much of his working life relating the surface anatomy of his patients to their deep structures (Fig. 1; see also Figs. 11 and 22). The following bony prominences can usually be palpated in the living subject (corresponding vertebral levels are given in brackets): •◊◊superior angle of the scapula (T2); •◊◊upper border of the manubrium sterni, the suprasternal notch (T2/3); •◊◊spine of the scapula (T3); •◊◊sternal angle (of Louis) — the transverse ridge at the manubrio-sternal junction (T4/5); •◊◊inferior angle of scapula (T8); •◊◊xiphisternal joint (T9); •◊◊lowest part of costal margin—10th rib (the subcostal line passes through L3). Note from Fig. 1 that the manubrium corresponds to the 3rd and 4th thoracic vertebrae and overlies the aortic arch, and that the sternum corre- sponds to the 5th to 8th vertebrae and neatly overlies the heart. Since the 1st and 12th ribs are difficult to feel, the ribs should be enu- merated from the 2nd costal cartilage, which articulates with the sternum at the angle of Louis. The spinous processes of all the thoracic vertebrae can be palpated in the midline posteriorly, but it should be remembered that the first spinous process that can be felt is that of C7 (the vertebra prominens). The position of the nipple varies considerably in the female, but in the male it usually lies in the 4th intercostal space about 4in (10cm) from the midline. -

Of the Pediatric Mediastinum
MRI of the Pediatric Mediastinum Dianna M. E. Bardo, MD Director of Body MR & Co-Director of the 3D Innovation Lab Disclosures Consultant & Speakers Bureau – honoraria Koninklijke Philips Healthcare N V Author – royalties Thieme Publishing Springer Publishing Mediastinum - Anatomy Superior Mediastinum thoracic inlet to thoracic plane thoracic plane to diaphragm Inferior Mediastinum lateral – pleural surface anterior – sternum posterior – vertebral bodies Mediastinum - Anatomy Anterior T4 Mediastinum pericardium to sternum Middle Mediastinum pericardial sac Posterior Mediastinum vertebral bodies to pericardium lateral – pleural surface superior – thoracic inlet inferior - diaphragm Mediastinum – MR Challenges Motion Cardiac ECG – gating/triggering Breathing Respiratory navigation Artifacts Intubation – LMA Surgical / Interventional materials Mediastinum – MR Sequences ECG gated/triggered sequences SSFP – black blood SE – IR – GRE Non- ECG gated/triggered sequences mDIXON (W, F, IP, OP), eTHRIVE, turbo SE, STIR, DWI Respiratory – triggered, radially acquired T2W MultiVane, BLADE, PROPELLER Mediastinum – MR Sequences MRA / MRV REACT – non Gd enhanced Gd enhanced sequences THRIVE, mDIXON, mDIXON XD Mediastinum – Contents Superior Mediastinum PVT Left BATTLE: Phrenic nerve Vagus nerve Structures at the level of the sternal angle Thoracic duct Left recurrent laryngeal nerve (not the right) CLAPTRAP Brachiocephalic veins Cardiac plexus Aortic arch (and its 3 branches) Ligamentum arteriosum Thymus Aortic arch (inner concavity) Trachea Pulmonary -

Thoracic Cage EDU - Module 2 > Thorax & Spine > Thorax & Spine
Thoracic Cage EDU - Module 2 > Thorax & Spine > Thorax & Spine Thoracic cage • Protects the chest organs (the heart and lungs). Main Structures: The sternum (aka, breastbone) lies anteriorly. 12 thoracic vertebrae lie posteriorly. 12 ribs articulate with the thoracic vertebrae. Sternum • Manubrium (superiorly) • Body (long and flat, middle portion) • Xiphoid process - Easily injured during chest compression (for CPR). • Sternal angle - Where manubrium and body meet - Easily palpated to find rib 2 • Sternal indentations: - Jugular notch (aka, suprasternal notch) is on the superior border of the manubrium. - Clavicular notches are to the sides of the jugular notch; these are where the clavicles (aka, collarbones), articulate with the sternum. - Costal notches articulate with the costal cartilages of the ribs ("costal" refers to the ribs). Rib Types • True ribs - Ribs 1-7; articulate with the sternum directly via their costal cartilages. • False ribs - Ribs 8-12; do not articulate directly with the sternum. - Ribs 11 and 12 are "floating ribs," do not articulate at all with the sternum. 1 / 2 Rib Features • Head - Articulates with the vertebral body; typically comprises two articular surfaces separated by a bony crest. • Neck - Extends from the head, and terminates at the tubercle. • Tubercle - Comprises an articular facet, which is where the rib articulates with the transverse process of the vertebra. • Shaft - Longest portion of the rib, extends from tubercle to rib end. • Angle - Bend in rib, just lateral to tubercle. Rib/vertebra articulation • Head and tubercle of rib articulate with body and thoracic process of vertebrae. Intercostal spaces • The spaces between the ribs • House muscles and neurovascular structures. -
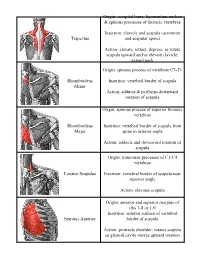
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Canine Thoracic Costovertebral and Costotransverse Joints Three Case Reports of Dysfunction and Manual Therapy Guidelines for A
Topics in Compan An Med 29 (2014) 1–5 Topical review Canine Thoracic Costovertebral and Costotransverse Joints: Three Case Reports of Dysfunction and Manual Therapy Guidelines for Assessment and Treatment of These Structures Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRTn Keywords: The costovertebral and costotransverse joints receive little attention in research. However, pain costovertebral associated with rib articulation dysfunction is reported to occur in human patients. The anatomic costotransverse structures of the canine rib joints and thoracic spine are similar to those of humans. As such, it is ribs physical therapy proposed that extrapolation from human physical therapy practice could be used for the assessment and rehabilitation treatment of the canine patient with presumed rib joint pain. This article presents 3 case studies that manual therapy demonstrate signs of rib dysfunction and successful treatment using primarily physical therapy manual techniques. General assessment and select treatment techniques are described. & 2014 Elsevier Inc. All rights reserved. The Canine Fitness Centre Ltd, Calgary, Alberta, Canada nAddress reprint requests to Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRT, The Canine Fitness Centre Ltd, 509—42nd Ave SE, Calgary, Alberta, Canada T2G 1Y7 E-mail: [email protected] The articular structures of the thorax comprise facet joints, the erect spine and further presented that in reviewing the literature, intervertebral disc, and costal joints. Little research has been they were unable to find mention of natural development of conducted on these joints in human or animal medicine. However, idiopathic scoliosis in quadrupeds; however, there are reports of clinical case presentations in human journals, manual therapy avian models and adolescent models in man. -

Required List of Bones and Markings
REQUIRED LIST OF BONES AND MARKINGS Axial Skeleton Skull Cranial Bones (8) Frontal Bone (1) Supraorbital foramina Supraorbital ridges or margins Parietal Bones (2) Temporal Bones (2) External auditory meatus Mastoid process Styloid process Zygomatic process Mandibular fossa Foramen lacerum Carotid foramen Jugular foramen Stylomastoid foramen Internal auditory meatus Occipital Bone (1) Foramen magnum Occipital condyles Ethmoid Bone (1) Cribriform plate Olfactory foramina in cribriform plate Crista galli Perpendicular plate (forms superior part of nasal septum) Middle nasal concha Superior nasal concha Sphenoid Bone (1) Foramen ovale Foramen rotundum Sella turcica Greater wing Lesser wing Optic foramen Inferior orbital fissure Superior orbital fissure Pterygoid processes Skull (cont’d) Facial Bones (14) Lacrimal Bones (2) Lacrimal fossa Nasal Bones (2) Inferior Nasal Conchae (2) Vomer (1) (forms inferior portion of nasal septum) Zygomatic Bones (2) Temporal process (forms zygomatic arch with zygomatic process of temporal bone) Maxillae (2) Alveoli Palatine process (forms anterior part of hard palate) Palatine Bones (2) (form posterior part of hard palate) Mandible (1) Alveoli Body Mental foramen Ramus Condylar process (mandibular condyle) Coronoid process Miscellaneous (Skull) Paranasal sinuses are located in the ethmoid bone, sphenoid bone, frontal bone, and maxillae Zygomatic arch (“cheekbone”) is composed of the zygomatic process of the temporal bone and the temporal process of the zygomatic bone 2 pairs of nasal conchae (superior and middle) are part of the ethmoid bone. 1 pair (inferior) are separate facial bones. All the scroll-like conchae project into the lateral walls of the nasal cavity. Hard palate (“roof of mouth”) is composed of 2 palatine processes of the maxillae and the 2 palatine bones (total of 4 fused bones). -

OMM PRACTICAL EXAM Saroj Misra, DO, FACOFP Rachel Nixon, DO Marissa Rogers, DO Family Medicine Goals/Objectives
OMM PRACTICAL EXAM Saroj Misra, DO, FACOFP Rachel Nixon, DO Marissa Rogers, DO Family Medicine Goals/Objectives • Review Exam Day procedure • Understand scoring process • Discuss possible cases and 2 OMM techniques that may be used for each case Disclaimer: The material being presented is NOT necessarily identical to what will be tested upon. We are not affiliated with the actual exam. This is our approach to the practical exam material. EXAM DAY Exam day • You will be assigned a time slot based on your last name • You will select a partner within your time slot • May not partner with a spouse or relative • You will be asked to sign a waiver stating that if you choose to do HVLA you will not perform the corrective “thrust” • You will then stand in line with your partner and await entering the testing room Exam day • There will be two rooms - one in which you will review cases and the second where you will be tested • Once you enter the first room you will not be able to leave • If you DO leave, both you and your partner will be given new cases Exam day • You will be given 3 cases: • Spine • Extremities • Systemic Disease • You will enter your name, ID number and your partners ID number on each case before turning them over Exam day • Each case will have the following information: • HPI • PMH • PSH • FHx • SocHx • There will be multiple choice for the best answer for your diagnosis • You will have 20 minutes to choose the best answer and plan a treatment strategy for each of your cases Exam day • After the 20 minutes are complete, you will