A Biomechanical Analysis of Ape and Human Thoracic Vertebrae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Structure and Function of Breathing
CHAPTERCONTENTS The structure-function continuum 1 Multiple Influences: biomechanical, biochemical and psychological 1 The structure and Homeostasis and heterostasis 2 OBJECTIVE AND METHODS 4 function of breathing NORMAL BREATHING 5 Respiratory benefits 5 Leon Chaitow The upper airway 5 Dinah Bradley Thenose 5 The oropharynx 13 The larynx 13 Pathological states affecting the airways 13 Normal posture and other structural THE STRUCTURE-FUNCTION considerations 14 Further structural considerations 15 CONTINUUM Kapandji's model 16 Nowhere in the body is the axiom of structure Structural features of breathing 16 governing function more apparent than in its Lung volumes and capacities 19 relation to respiration. This is also a region in Fascla and resplrstory function 20 which prolonged modifications of function - Thoracic spine and ribs 21 Discs 22 such as the inappropriate breathing pattern dis- Structural features of the ribs 22 played during hyperventilation - inevitably intercostal musculature 23 induce structural changes, for example involving Structural features of the sternum 23 Posterior thorax 23 accessory breathing muscles as well as the tho- Palpation landmarks 23 racic articulations. Ultimately, the self-perpetuat- NEURAL REGULATION OF BREATHING 24 ing cycle of functional change creating structural Chemical control of breathing 25 modification leading to reinforced dysfunctional Voluntary control of breathing 25 tendencies can become complete, from The autonomic nervous system 26 whichever direction dysfunction arrives, for Sympathetic division 27 Parasympathetic division 27 example: structural adaptations can prevent NANC system 28 normal breathing function, and abnormal breath- THE MUSCLES OF RESPIRATION 30 ing function ensures continued structural adap- Additional soft tissue influences and tational stresses leading to decompensation. -

Vertebral Column and Thorax
Introduction to Human Osteology Chapter 4: Vertebral Column and Thorax Roberta Hall Kenneth Beals Holm Neumann Georg Neumann Gwyn Madden Revised in 1978, 1984, and 2008 The Vertebral Column and Thorax Sternum Manubrium – bone that is trapezoidal in shape, makes up the superior aspect of the sternum. Jugular notch – concave notches on either side of the superior aspect of the manubrium, for articulation with the clavicles. Corpus or body – flat, rectangular bone making up the major portion of the sternum. The lateral aspects contain the notches for the true ribs, called the costal notches. Xiphoid process – variably shaped bone found at the inferior aspect of the corpus. Process may fuse late in life to the corpus. Clavicle Sternal end – rounded end, articulates with manubrium. Acromial end – flat end, articulates with scapula. Conoid tuberosity – muscle attachment located on the inferior aspect of the shaft, pointing posteriorly. Ribs Scapulae Head Ventral surface Neck Dorsal surface Tubercle Spine Shaft Coracoid process Costal groove Acromion Glenoid fossa Axillary margin Medial angle Vertebral margin Manubrium. Left anterior aspect, right posterior aspect. Sternum and Xyphoid Process. Left anterior aspect, right posterior aspect. Clavicle. Left side. Top superior and bottom inferior. First Rib. Left superior and right inferior. Second Rib. Left inferior and right superior. Typical Rib. Left inferior and right superior. Eleventh Rib. Left posterior view and left superior view. Twelfth Rib. Top shows anterior view and bottom shows posterior view. Scapula. Left side. Top anterior and bottom posterior. Scapula. Top lateral and bottom superior. Clavicle Sternum Scapula Ribs Vertebrae Body - Development of the vertebrae can be used in aging of individuals. -
Part 1 the Thorax ECA1 7/18/06 6:30 PM Page 2 ECA1 7/18/06 6:30 PM Page 3
ECA1 7/18/06 6:30 PM Page 1 Part 1 The Thorax ECA1 7/18/06 6:30 PM Page 2 ECA1 7/18/06 6:30 PM Page 3 Surface anatomy and surface markings The experienced clinician spends much of his working life relating the surface anatomy of his patients to their deep structures (Fig. 1; see also Figs. 11 and 22). The following bony prominences can usually be palpated in the living subject (corresponding vertebral levels are given in brackets): •◊◊superior angle of the scapula (T2); •◊◊upper border of the manubrium sterni, the suprasternal notch (T2/3); •◊◊spine of the scapula (T3); •◊◊sternal angle (of Louis) — the transverse ridge at the manubrio-sternal junction (T4/5); •◊◊inferior angle of scapula (T8); •◊◊xiphisternal joint (T9); •◊◊lowest part of costal margin—10th rib (the subcostal line passes through L3). Note from Fig. 1 that the manubrium corresponds to the 3rd and 4th thoracic vertebrae and overlies the aortic arch, and that the sternum corre- sponds to the 5th to 8th vertebrae and neatly overlies the heart. Since the 1st and 12th ribs are difficult to feel, the ribs should be enu- merated from the 2nd costal cartilage, which articulates with the sternum at the angle of Louis. The spinous processes of all the thoracic vertebrae can be palpated in the midline posteriorly, but it should be remembered that the first spinous process that can be felt is that of C7 (the vertebra prominens). The position of the nipple varies considerably in the female, but in the male it usually lies in the 4th intercostal space about 4in (10cm) from the midline. -

Chapter 21 Fractures of the Upper Thoracic Spine: Approaches and Surgical Management
Chapter 21 Fractures of the Upper Thoracic Spine: Approaches and Surgical Management Sean D Christie, M.D., John Song, M.D., and Richard G Fessler, M.D., Ph.D. INTRODUCTION Fractures occurring in the thoracic region account for approximately 17 to 23% of all traumatic spinal fractures (1), with 22% of traumatic spinal fractures occurring between T1 and T4 (16). More than half of these fractures result in neurological injury, and almost three-quarters of those impaired suffer from complete paralysis. Obtaining surgical access to the anterior vertebral elements of the upper thoracic vertebrae (T1–T6) presents a unique anatomic challenge. The thoracic cage, which narrows significantly as it approaches the thoracic inlet, has an intimate association between the vertebral column and the superior mediastinal structures. The supraclavicular, transmanubrial, transthoracic, and lateral parascapular extrapleural approaches each provide access to the anterior vertebral elements of the upper thoracic vertebrae. However, each of these approaches has distinct advantages and disadvantages and their use should be tailored to each individual patient’s situation. This chapter reviews these surgical approaches. Traditional posterior approaches are illustrated in Figure 21.1, but will not be discussed in depth here. ANATOMIC CONSIDERATIONS AND STABILITY The upper thoracic spine possesses unique anatomic and biomechanical properties. The anterior aspects of the vertebral bodies are smaller than the posterior aspects, which contribute to the physiological kyphosis present in this region of the spine. Furthermore, this orientation results in a ventrally positioned axis of rotation, predisposing this region to compression injuries. The combination and interaction of the vertebral bodies, ribs, and sternum increase the inherent biomechanical stability of this segment of the spine to 2 to 3 times that of the thoracolumbar junction. -

Thoracic Cage EDU - Module 2 > Thorax & Spine > Thorax & Spine
Thoracic Cage EDU - Module 2 > Thorax & Spine > Thorax & Spine Thoracic cage • Protects the chest organs (the heart and lungs). Main Structures: The sternum (aka, breastbone) lies anteriorly. 12 thoracic vertebrae lie posteriorly. 12 ribs articulate with the thoracic vertebrae. Sternum • Manubrium (superiorly) • Body (long and flat, middle portion) • Xiphoid process - Easily injured during chest compression (for CPR). • Sternal angle - Where manubrium and body meet - Easily palpated to find rib 2 • Sternal indentations: - Jugular notch (aka, suprasternal notch) is on the superior border of the manubrium. - Clavicular notches are to the sides of the jugular notch; these are where the clavicles (aka, collarbones), articulate with the sternum. - Costal notches articulate with the costal cartilages of the ribs ("costal" refers to the ribs). Rib Types • True ribs - Ribs 1-7; articulate with the sternum directly via their costal cartilages. • False ribs - Ribs 8-12; do not articulate directly with the sternum. - Ribs 11 and 12 are "floating ribs," do not articulate at all with the sternum. 1 / 2 Rib Features • Head - Articulates with the vertebral body; typically comprises two articular surfaces separated by a bony crest. • Neck - Extends from the head, and terminates at the tubercle. • Tubercle - Comprises an articular facet, which is where the rib articulates with the transverse process of the vertebra. • Shaft - Longest portion of the rib, extends from tubercle to rib end. • Angle - Bend in rib, just lateral to tubercle. Rib/vertebra articulation • Head and tubercle of rib articulate with body and thoracic process of vertebrae. Intercostal spaces • The spaces between the ribs • House muscles and neurovascular structures. -
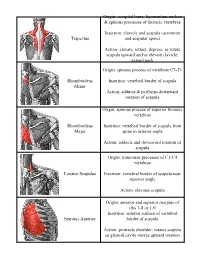
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Required List of Bones and Markings
REQUIRED LIST OF BONES AND MARKINGS Axial Skeleton Skull Cranial Bones (8) Frontal Bone (1) Supraorbital foramina Supraorbital ridges or margins Parietal Bones (2) Temporal Bones (2) External auditory meatus Mastoid process Styloid process Zygomatic process Mandibular fossa Foramen lacerum Carotid foramen Jugular foramen Stylomastoid foramen Internal auditory meatus Occipital Bone (1) Foramen magnum Occipital condyles Ethmoid Bone (1) Cribriform plate Olfactory foramina in cribriform plate Crista galli Perpendicular plate (forms superior part of nasal septum) Middle nasal concha Superior nasal concha Sphenoid Bone (1) Foramen ovale Foramen rotundum Sella turcica Greater wing Lesser wing Optic foramen Inferior orbital fissure Superior orbital fissure Pterygoid processes Skull (cont’d) Facial Bones (14) Lacrimal Bones (2) Lacrimal fossa Nasal Bones (2) Inferior Nasal Conchae (2) Vomer (1) (forms inferior portion of nasal septum) Zygomatic Bones (2) Temporal process (forms zygomatic arch with zygomatic process of temporal bone) Maxillae (2) Alveoli Palatine process (forms anterior part of hard palate) Palatine Bones (2) (form posterior part of hard palate) Mandible (1) Alveoli Body Mental foramen Ramus Condylar process (mandibular condyle) Coronoid process Miscellaneous (Skull) Paranasal sinuses are located in the ethmoid bone, sphenoid bone, frontal bone, and maxillae Zygomatic arch (“cheekbone”) is composed of the zygomatic process of the temporal bone and the temporal process of the zygomatic bone 2 pairs of nasal conchae (superior and middle) are part of the ethmoid bone. 1 pair (inferior) are separate facial bones. All the scroll-like conchae project into the lateral walls of the nasal cavity. Hard palate (“roof of mouth”) is composed of 2 palatine processes of the maxillae and the 2 palatine bones (total of 4 fused bones). -

Vertebral Column
Vertebral Column • Backbone consists of Cervical 26 vertebrae. • Five vertebral regions – Cervical vertebrae (7) Thoracic in the neck. – Thoracic vertebrae (12) in the thorax. – Lumbar vertebrae (5) in the lower back. Lumbar – Sacrum (5, fused). – Coccyx (4, fused). Sacrum Coccyx Scoliosis Lordosis Kyphosis Atlas (C1) Posterior tubercle Vertebral foramen Tubercle for transverse ligament Superior articular facet Transverse Transverse process foramen Facet for dens Anterior tubercle • Atlas- ring of bone, superior facets for occipital condyles. – Nodding movement signifies “yes”. Axis (C2) Spinous process Lamina Vertebral foramen Transverse foramen Transverse process Superior articular facet Odontoid process (dens) •Axis- dens or odontoid process is body of atlas. – Pivotal movement signifies “no”. Typical Cervical Vertebra (C3-C7) • Smaller bodies • Larger spinal canal • Transverse processes –Shorter – Transverse foramen for vertebral artery • Spinous processes of C2 to C6 often bifid • 1st and 2nd cervical vertebrae are unique – Atlas & axis Typical Cervical Vertebra Spinous process (bifid) Lamina Vertebral foramen Inferior articular process Superior articular process Transverse foramen Pedicle Transverse process Body Thoracic Vertebrae (T1-T12) • Larger and stronger bodies • Longer transverse & spinous processes • Demifacets on body for head of rib • Facets on transverse processes (T1-T10) for tubercle of rib Thoracic Vertebra- superior view Spinous process Transverse process Facet for tubercle of rib Lamina Superior articular process -

Rate of Presence of 11 Thoracic Vertebrae and 6 Lumbar Vertebrae In
Yan et al. Journal of Orthopaedic Surgery and Research (2018) 13:124 https://doi.org/10.1186/s13018-018-0835-9 RESEARCHARTICLE Open Access Rate of presence of 11 thoracic vertebrae and 6 lumbar vertebrae in asymptomatic Chinese adult volunteers Ying-zhao Yan1, Qing-ping Li2, Cong-cong Wu1, Xiang-xiang Pan1, Zhen-xuan Shao1, Shao-qing Chen2, Ke Wang1, Xi-bang Chen1 and Xiang-yang Wang1* Abstract Background: An increasing number of studies on spinal morphology in asymptomatic Asian and Western patients have been reported. Variation in spinal anatomy among patients is considered as the cause of wrong-level surgery in up to 40% of cases. The present study examined the rate of presence of 11 thoracic vertebrae and 6 lumbar vertebrae in 293 asymptomatic Chinese adult volunteers. Methods: From May 27, 2016, to November 11, 2017, a cohort of 325 asymptomatic Chinese adults meeting the study exclusion criteria was recruited. The radiographs were examined by a spine surgeon and a radiologist to assess the number of thoracic and lumbar vertebrae. Results: In total, 293 volunteers were included in this study: 17 (5.8%) had 11 thoracic vertebrae, and 16 (5.5%) had 6 lumbar vertebrae. Among all volunteers, 12 (4.1%) had 7 cervical vertebrae (C), 11 thoracic vertebrae (T), and 5 lumbar vertebrae (L); 5 (1.7%) had 7C, 11T, and 6L; and 11 (3.8%) had 7C, 12T, and 6L. There was no difference between the findings of the spine surgeon and the radiologist. Conclusions: For the first time, this study describes the rate of presence of 11 thoracic vertebrae and 6 lumbar vertebrae in 293 asymptomatic Chinese adult volunteers. -

Cervical Vertebrae 1 Cervical Vertebrae
Cervical vertebrae 1 Cervical vertebrae Cervical vertebrae or Cervilar Position of human cervical vertebrae (shown in red). It consists of 7 bones, from top to bottom, C1, C2, C3, C4, C5, C6 and C7. A human cervical vertebra Latin Vertebrae cervicales [1] Gray's p.97 [2] MeSH Cervical+vertebrae [3] TA A02.2.02.001 [4] FMA FMA:72063 In vertebrates, cervical vertebrae (singular: vertebra) are those vertebrae immediately inferior to the skull. Thoracic vertebrae in all mammalian species are defined as those vertebrae that also carry a pair of ribs, and lie caudal to the cervical vertebrae. Further caudally follow the lumbar vertebrae, which also belong to the trunk, but do not carry ribs. In reptiles, all trunk vertebrae carry ribs and are called dorsal vertebrae. In many species, though not in mammals, the cervical vertebrae bear ribs. In many other groups, such as lizards and saurischian dinosaurs, the cervical ribs are large; in birds, they are small and completely fused to the vertebrae. The transverse processes of mammals are homologous to the cervical ribs of other amniotes. Cervical vertebrae 2 In humans, cervical vertebrae are the smallest of the true vertebrae, and can be readily distinguished from those of the thoracic or lumbar regions by the presence of a foramen (hole) in each transverse process, through which passes the vertebral artery. The remainder of this article focuses upon human anatomy. Structure By convention, the cervical vertebrae are numbered, with the first one (C1) located closest to the skull and higher numbered vertebrae (C2-C7) proceeding away from the skull and down the spine. -

Sonographic Tracking of Trunk Nerves: Essential for Ultrasound-Guided Pain Management and Research
Journal name: Journal of Pain Research Article Designation: Perspectives Year: 2017 Volume: 10 Journal of Pain Research Dovepress Running head verso: Chang et al Running head recto: Sonographic tracking of trunk nerve open access to scientific and medical research DOI: http://dx.doi.org/10.2147/JPR.S123828 Open Access Full Text Article PERSPECTIVES Sonographic tracking of trunk nerves: essential for ultrasound-guided pain management and research Ke-Vin Chang1,2 Abstract: Delineation of architecture of peripheral nerves can be successfully achieved by Chih-Peng Lin2,3 high-resolution ultrasound (US), which is essential for US-guided pain management. There Chia-Shiang Lin4,5 are numerous musculoskeletal pain syndromes involving the trunk nerves necessitating US for Wei-Ting Wu1 evaluation and guided interventions. The most common peripheral nerve disorders at the trunk Manoj K Karmakar6 region include thoracic outlet syndrome (brachial plexus), scapular winging (long thoracic nerve), interscapular pain (dorsal scapular nerve), and lumbar facet joint syndrome (medial branches Levent Özçakar7 of spinal nerves). Until now, there is no single article systematically summarizing the anatomy, 1 Department of Physical Medicine sonographic pictures, and video demonstration of scanning techniques regarding trunk nerves. and Rehabilitation, National Taiwan University Hospital, Bei-Hu Branch, In this review, the authors have incorporated serial figures of transducer placement, US images, Taipei, Taiwan; 2National Taiwan and videos for scanning the nerves in the trunk region and hope this paper helps physicians University College of Medicine, familiarize themselves with nerve sonoanatomy and further apply this technique for US-guided Taipei, Taiwan; 3Department of Anesthesiology, National Taiwan pain medicine and research. -

Bones of the Trunk
BONES OF THE TRUNK Andrea Heinzlmann Veterinary University Department of Anatomy and Histology 16th September 2019 VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) • the vertebral column composed of the vertebrae • the vertebrae form a horizontal chain https://hu.pinterest.com/pin/159877855502035893/ VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) along the vertebral column three major curvatures are recognized: 1. the DORSAL CONVEX CURVATURE – between the head and the neck 2. the DORSAL CONCAVE CURVATURE – between the neck and the chest 3. the DORSAL CONVEX CURVATURE – between the thorax and the lumbar region - in carnivores (Ca) there is an additional DORSAL CONVEXITY in the sacral region https://hu.pinterest.com/pin/159877855502035893/ VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) - corresponding to the regions of the body, we distinguish: 1. CERVICAL VERTEBRAE 2. THORACIC VERTEBRAE 3. LUMBAR VERTEBRAE 4. SACRAL VERTEBRAE 5. CAUDAL (COCCYGEAL) VERTEBRAE https://www.ufaw.org.uk/dogs/french-bulldog-hemivertebrae https://rogueshock.com/know-your-horse-in-9-ways/5/ BUILD OF THE VERTEBRAE each vertebrae presents: 1. BODY (CORPUS VERTEBRAE) 2. ARCH (ARCUS VERTEBRAE) 3. PROCESSES corpus Vertebra thoracica (Th13) , Ca. THE VERTEBRAL BODY (CORPUS VERTEBRAE) - the ventral portion of the vertebra ITS PARTS: 1. EXTREMITAS CRANIALIS (seu CAPUT VERTEBRAE) – convex 2. EXTREMITAS CAUDALIS (seu FOSSA VERTEBRAE) - concave Th13, Ca. THE VERTEBRAL BODY (CORPUS VERTEBRAE) 3. VENTRAL SURFACE of the body has a: - ventral crest (CRISTA VENTRALIS) 4. DORSAL SURFACE of the body carries : - the vertebral arch (ARCUS VERTEBRAE) Th13, Ca., lateral aspect Arcus vertebrae corpus Vertebra thoracica (Th13) , Ca., caudal aspect THE VERTEBRAL BODY (CORPUS VERTEBRAE) 6. VERTEBRAL ARCH (ARCUS VERTEBRAE) compraisis: a) a ventral PEDICULUS ARCUS VERTEBRAE b) a dorsal LAMINA ARCUS VERTEBRAE C7, Ca.