The Unified Myofibrillar Matrix for Force Generation in Muscle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microanatomy of Muscles
Microanatomy of Muscles Anatomy & Physiology Class Three Main Muscle Types Objectives: By the end of this presentation you will have the information to: 1. Describe the 3 main types of muscles. 2. Detail the functions of the muscle system. 3. Correctly label the parts of a myocyte (muscle cell) 4. Identify the levels of organization in a skeletal muscle from organ to myosin. 5. Explain how a muscle contracts utilizing the correct terminology of the sliding filament theory. 6. Contrast and compare cardiac and smooth muscle with skeletal muscle. Major Functions: Muscle System 1. Moving the skeletal system and posture. 2. Passing food through the digestive system & constriction of other internal organs. 3. Production of body heat. 4. Pumping the blood throughout the body. 5. Communication - writing and verbal Specialized Cells (Myocytes) ~ Contractile Cells Can shorten along one or more planes because of specialized cell membrane (sarcolemma) and specialized cytoskeleton. Specialized Structures found in Myocytes Sarcolemma: The cell membrane of a muscle cell Transverse tubule: a tubular invagination of the sarcolemma of skeletal or cardiac muscle fibers that surrounds myofibrils; involved in transmitting the action potential from the sarcolemma to the interior of the myofibril. Sarcoplasmic Reticulum: The special type of smooth endoplasmic Myofibrils: reticulum found in smooth and a contractile fibril of skeletal muscle, composed striated muscle fibers whose function mainly of actin and myosin is to store and release calcium ions. Multiple Nuclei (skeletal) & many mitochondria Skeletal Muscle - Microscopic Anatomy A whole skeletal muscle (such as the biceps brachii) is considered an organ of the muscular system. Each organ consists of skeletal muscle tissue, connective tissue, nerve tissue, and blood or vascular tissue. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

Muscle Histology
Muscle Histology Dr. Heba Kalbouneh Functions of muscle tissue ▪ Movement ▪ Maintenance of posture ▪ Joint stabilization ▪ Heat generation Types of Muscle Tissue ▪ Skeletal muscle ▪ Cardiac muscle ▪ Smooth muscle Types of Muscle Tissue Skeletal •Attach to and move skeleton •40% of body weight •Fibers = multinucleate cells (embryonic cells fuse) •Cells with obvious striations •Contractions are voluntary Cardiac: only in the wall of the heart •Cells are striated •Contractions are involuntary (not voluntary) Smooth: walls of hollow organs •Lack striations •Contractions are involuntary (not voluntary) Similarities… ▪ Their cells are called fibers because they are elongated ▪ Contraction depends on myofilaments ▪ Actin ▪ Myosin ▪ Plasma membrane is called sarcolemma ▪ Sarcos = flesh ▪ Lemma = sheath SKELETAL MUSCLES Epimysium: surrounds whole muscle Endomysium is around each muscle fiber Perimysium is around fascicle = muscle cell= myofiber Skeletal muscle This big cylinder is a fiber: a cell ▪ Fibers (each is one cell) have striations ▪ Myofibrils are organelles of the cell: these are made -an organelle up of myofilaments ▪ Sarcomere ▪ Basic unit of contraction ▪ Myofibrils are long rows of repeating sarcomeres ▪ Boundaries: Z discs (or lines) Sarcomere M line provides an attachment to myosin filaments Z line provides an attachment to actin filaments A band is the darker band of the myofibril containing myosin filaments H band is the lighter section in the middle of the A band where only myosin is present I band is the lighter band containing -

(7E) Powerpoint Lecture Outline Chapter 8: Control of Movement
Carlson (7e) PowerPoint Lecture Outline Chapter 8: Control of Movement This multimedia product and its contents are protected under copyright law. The following are prohibited by law: •any public performance or display, including transmission of any image over a network; •preparation of any derivative work, including extraction, in whole or in part, of any images; •any rental, lease, or lending of the program. Copyright 2001 by Allyn & Bacon Skeletal Muscle n Movements of our body are accomplished by contraction of the skeletal muscles l Flexion: contraction of a flexor muscle draws in a limb l Extension: contraction of extensor muscle n Skeletal muscle fibers have a striated appearance n Skeletal muscle is composed of two fiber types: l Extrafusal: innervated by alpha-motoneurons from the spinal cord: exert force l Intrafusal: sensory fibers that detect stretch of the muscle u Afferent fibers: report length of intrafusal: when stretched, the fibers stimulate the alpha-neuron that innervates the muscle fiber: maintains muscle tone u Efferent fibers: contraction adjusts sensitivity of afferent fibers. 8.2 Copyright 2001 by Allyn & Bacon Skeletal Muscle Anatomy n Each muscle fiber consists of a bundle of myofibrils l Each myofibril is made up of overlapping strands of actin and myosin l During a muscle twitch, the myosin filaments move relative to the actin filaments, thereby shortening the muscle fiber 8.3 Copyright 2001 by Allyn & Bacon Neuromuscular Junction n The neuromuscular junction is the synapse formed between an alpha motor neuron -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
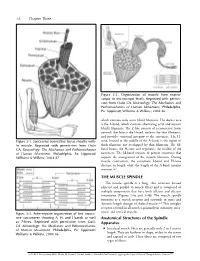
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

The Role of the Actin Cytoskeleton During Muscle Development In
THE ROLE OF THE ACTIN CYTOSKELETON DURING MUSCLE DEVELOPMENT IN DROSOPHILA AND MOUSE by Shannon Faye Yu A Dissertation Presented to the Faculty of the Louis V. Gerstner, Jr. Graduate School of the Biomedical Sciences in Partial Fulfillment of the Requirements of the Degree of Doctor of Philosophy New York, NY Oct, 2013 Mary K. Baylies, PhD! Date Dissertation Mentor Copyright by Shannon F. Yu 2013 ABSTRACT The actin cytoskeleton is essential for many processes within a developing organism. Unsurprisingly, actin and its regulators underpin many of the critical steps in the formation and function of muscle tissue. These include cell division during the specification of muscle progenitors, myoblast fusion, muscle elongation and attachment, and muscle maturation, including sarcomere assembly. Analysis in Drosophila has focused on regulators of actin polymerization particularly during myoblast fusion, and the conservation of many of the actin regulators required for muscle development has not yet been tested. In addition, dynamic actin processes also require the depolymerization of existing actin fibers to replenish the pool of actin monomers available for polymerization. Despite this, the role of actin depolymerization has not been described in depth in Drosophila or mammalian muscle development. ! Here, we first examine the role of the actin depolymerization factor Twinstar (Tsr) in muscle development in Drosophila. We show that Twinstar, the sole Drosophila member of the ADF/cofilin family of actin depolymerization proteins, is expressed in muscle where it is essential for development. tsr mutant embryos displayed a number of muscle defects, including muscle loss and muscle misattachment. Further, regulators of Tsr, including a Tsr-inactivating kinase, Center divider, a Tsr-activating phosphatase, Slingshot and a synergistic partner in depolymerization, Flare, are also required for embryonic muscle development. -

CAP2 Deficiency Delays Myofibril Actin Cytoskeleton Differentiation and Disturbs Skeletal Muscle Architecture and Function
CAP2 deficiency delays myofibril actin cytoskeleton differentiation and disturbs skeletal muscle architecture and function Lara-Jane Kepsera, Fidan Damara, Teresa De Ciccob, Christine Chaponnierc, Tomasz J. Prószynski b, Axel Pagenstecherd, and Marco B. Rusta,e,f,1 aMolecular Neurobiology Group, Institute of Physiological Chemistry, University of Marburg, 35032 Marburg, Germany; bLaboratory of Synaptogenesis, Nencki Institute of Experimental Biology PAS, 02-093 Warsaw, Poland; cDepartment of Pathology and Immunology, University of Geneva, 1211 Geneva, Switzerland; dInstitute of Neuropathology, University of Marburg, 35032 Marburg, Germany; eCenter for Mind, Brain and Behavior, Research Campus of Central Hessen, 35032 Marburg, Germany; and fDFG Research Training Group “Membrane Plasticity in Tissue Development and Remodeling,” GRK 2213, University of Marburg, 35032 Marburg, Germany Edited by Yale E. Goldman, University of Pennsylvania/PMI, Philadelphia, PA, and approved March 14, 2019 (received for review August 7, 2018) Actin filaments (F-actin) are key components of sarcomeres, the have acquired specific functions. While previous analyses of mu- basic contractile units of skeletal muscle myofibrils. A crucial step tant mice demonstrated a role of CAP2 in neuron morphology and during myofibril differentiation is the sequential exchange of heart physiology (13–15), its function in skeletal muscles has not α-actin isoforms from smooth muscle (α-SMA) and cardiac (α-CAA) been investigated, yet. to skeletal muscle α-actin (α-SKA) that, in mice, occurs during early We here report a function for CAP2 in skeletal muscle de- postnatal life. This “α-actin switch” requires the coordinated activ- velopment. We found that CAP2 controls the exchange of ity of actin regulators because it is vital that sarcomere structure α-actin isoforms during myofibril differentiation. -

Muscle Physiology Dr
Muscle Physiology Dr. Ebneshahidi Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Skeletal Muscle Figure 9.2 (a) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Functions of the muscular system . 1. Locomotion . 2. Vasoconstriction and vasodilatation- constriction and dilation of blood vessel Walls are the results of smooth muscle contraction. 3. Peristalsis – wavelike motion along the digestive tract is produced by the Smooth muscle. 4. Cardiac motion . 5. Posture maintenance- contraction of skeletal muscles maintains body posture and muscle tone. 6. Heat generation – about 75% of ATP energy used in muscle contraction is released as heat. Copyright. © 2004 Pearson Education, Inc., publishing as Benjamin Cummings . Striation: only present in skeletal and cardiac muscles. Absent in smooth muscle. Nucleus: smooth and cardiac muscles are uninculcated (one nucleus per cell), skeletal muscle is multinucleated (several nuclei per cell ). Transverse tubule ( T tubule ): well developed in skeletal and cardiac muscles to transport calcium. Absent in smooth muscle. Intercalated disk: specialized intercellular junction that only occurs in cardiac muscle. Control: skeletal muscle is always under voluntary control‚ with some exceptions ( the tongue and pili arrector muscles in the dermis). smooth and cardiac muscles are under involuntary control. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Innervation: motor unit . a) a motor nerve and a myofibril from a neuromuscular junction where gap (called synapse) occurs between the two structures. at the end of motor nerve‚ neurotransmitter (i.e. acetylcholine) is stored in synaptic vesicles which will release the neurotransmitter using exocytosis upon the stimulation of a nerve impulse. Across the synapse the surface the of myofibril contains receptors that can bind with the neurotransmitter. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Uniform Sarcomere Shortening Behavior in Isolated Cardiac Muscle Cells
Uniform Sarcomere Shortening Behavior in Isolated Cardiac Muscle Cells JOHN W. KRUEGER, DAMIAN FORLETTI, and BEATRICE A. WITTENBERG From the Albert Einstein College of Medicine, Departments of Medicine and Physiology, Bronx, New York 10461 ABSTRACT We have observed the dynamics of sarcomere shortening and the diffracting action of single, functionally intact, unattached cardiac muscle cells enzymatically isolated from the ventricular tissue of adult rats. Sarcomere length was measured either (a) continuously by a light diffraction method or (b) by direct inspection of the cell's striated image as recorded on videotape or by cinemicroscopy (120-400 frames/s). At physiological levels of added CaCl2 (0.5- 2.0 mM), many cells were quiescent (i.e., they did not beat spontaneously) and contracted in response to electrical stimulation (~ 1.0-ms pulse width). Sarcomere length in the quiescent, unstimulated cells (1.93 + 0.10 [SD] /~m), at peak shortening (1.57 + 0.13/~m, n = 49), and the maximum velocity of sarcomere shortening and relengthening were comparable to previous observations in intact heart muscle preparations. The dispersion of light diffracted by the cell remained narrow, and individual striations remained distinct and laterally well registered throughout the shortening-relengthening cycle. In contrast, apprecia- ble nonuniformity and internal buckling were seen at sarcomere lengths < 1.8 /~m when the resting cell, embedded in gelatin, was longitudinally compressed. These results indicate (a) that shortening and relengthening is characterized by uniform activation between myofibrils within the cardiac cell and (b) that physiologically significant relengthening forces in living heart muscle originate at the level of the cell rather than in extracellular connections.