Microfluidic Perfusion Shows Intersarcomere Dynamics Within Single Skeletal Muscle Myofibrils
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microanatomy of Muscles
Microanatomy of Muscles Anatomy & Physiology Class Three Main Muscle Types Objectives: By the end of this presentation you will have the information to: 1. Describe the 3 main types of muscles. 2. Detail the functions of the muscle system. 3. Correctly label the parts of a myocyte (muscle cell) 4. Identify the levels of organization in a skeletal muscle from organ to myosin. 5. Explain how a muscle contracts utilizing the correct terminology of the sliding filament theory. 6. Contrast and compare cardiac and smooth muscle with skeletal muscle. Major Functions: Muscle System 1. Moving the skeletal system and posture. 2. Passing food through the digestive system & constriction of other internal organs. 3. Production of body heat. 4. Pumping the blood throughout the body. 5. Communication - writing and verbal Specialized Cells (Myocytes) ~ Contractile Cells Can shorten along one or more planes because of specialized cell membrane (sarcolemma) and specialized cytoskeleton. Specialized Structures found in Myocytes Sarcolemma: The cell membrane of a muscle cell Transverse tubule: a tubular invagination of the sarcolemma of skeletal or cardiac muscle fibers that surrounds myofibrils; involved in transmitting the action potential from the sarcolemma to the interior of the myofibril. Sarcoplasmic Reticulum: The special type of smooth endoplasmic Myofibrils: reticulum found in smooth and a contractile fibril of skeletal muscle, composed striated muscle fibers whose function mainly of actin and myosin is to store and release calcium ions. Multiple Nuclei (skeletal) & many mitochondria Skeletal Muscle - Microscopic Anatomy A whole skeletal muscle (such as the biceps brachii) is considered an organ of the muscular system. Each organ consists of skeletal muscle tissue, connective tissue, nerve tissue, and blood or vascular tissue. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

(7E) Powerpoint Lecture Outline Chapter 8: Control of Movement
Carlson (7e) PowerPoint Lecture Outline Chapter 8: Control of Movement This multimedia product and its contents are protected under copyright law. The following are prohibited by law: •any public performance or display, including transmission of any image over a network; •preparation of any derivative work, including extraction, in whole or in part, of any images; •any rental, lease, or lending of the program. Copyright 2001 by Allyn & Bacon Skeletal Muscle n Movements of our body are accomplished by contraction of the skeletal muscles l Flexion: contraction of a flexor muscle draws in a limb l Extension: contraction of extensor muscle n Skeletal muscle fibers have a striated appearance n Skeletal muscle is composed of two fiber types: l Extrafusal: innervated by alpha-motoneurons from the spinal cord: exert force l Intrafusal: sensory fibers that detect stretch of the muscle u Afferent fibers: report length of intrafusal: when stretched, the fibers stimulate the alpha-neuron that innervates the muscle fiber: maintains muscle tone u Efferent fibers: contraction adjusts sensitivity of afferent fibers. 8.2 Copyright 2001 by Allyn & Bacon Skeletal Muscle Anatomy n Each muscle fiber consists of a bundle of myofibrils l Each myofibril is made up of overlapping strands of actin and myosin l During a muscle twitch, the myosin filaments move relative to the actin filaments, thereby shortening the muscle fiber 8.3 Copyright 2001 by Allyn & Bacon Neuromuscular Junction n The neuromuscular junction is the synapse formed between an alpha motor neuron -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
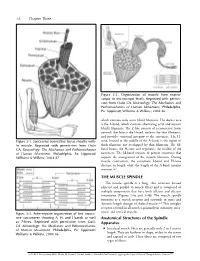
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

CAP2 Deficiency Delays Myofibril Actin Cytoskeleton Differentiation and Disturbs Skeletal Muscle Architecture and Function
CAP2 deficiency delays myofibril actin cytoskeleton differentiation and disturbs skeletal muscle architecture and function Lara-Jane Kepsera, Fidan Damara, Teresa De Ciccob, Christine Chaponnierc, Tomasz J. Prószynski b, Axel Pagenstecherd, and Marco B. Rusta,e,f,1 aMolecular Neurobiology Group, Institute of Physiological Chemistry, University of Marburg, 35032 Marburg, Germany; bLaboratory of Synaptogenesis, Nencki Institute of Experimental Biology PAS, 02-093 Warsaw, Poland; cDepartment of Pathology and Immunology, University of Geneva, 1211 Geneva, Switzerland; dInstitute of Neuropathology, University of Marburg, 35032 Marburg, Germany; eCenter for Mind, Brain and Behavior, Research Campus of Central Hessen, 35032 Marburg, Germany; and fDFG Research Training Group “Membrane Plasticity in Tissue Development and Remodeling,” GRK 2213, University of Marburg, 35032 Marburg, Germany Edited by Yale E. Goldman, University of Pennsylvania/PMI, Philadelphia, PA, and approved March 14, 2019 (received for review August 7, 2018) Actin filaments (F-actin) are key components of sarcomeres, the have acquired specific functions. While previous analyses of mu- basic contractile units of skeletal muscle myofibrils. A crucial step tant mice demonstrated a role of CAP2 in neuron morphology and during myofibril differentiation is the sequential exchange of heart physiology (13–15), its function in skeletal muscles has not α-actin isoforms from smooth muscle (α-SMA) and cardiac (α-CAA) been investigated, yet. to skeletal muscle α-actin (α-SKA) that, in mice, occurs during early We here report a function for CAP2 in skeletal muscle de- postnatal life. This “α-actin switch” requires the coordinated activ- velopment. We found that CAP2 controls the exchange of ity of actin regulators because it is vital that sarcomere structure α-actin isoforms during myofibril differentiation. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Uniform Sarcomere Shortening Behavior in Isolated Cardiac Muscle Cells
Uniform Sarcomere Shortening Behavior in Isolated Cardiac Muscle Cells JOHN W. KRUEGER, DAMIAN FORLETTI, and BEATRICE A. WITTENBERG From the Albert Einstein College of Medicine, Departments of Medicine and Physiology, Bronx, New York 10461 ABSTRACT We have observed the dynamics of sarcomere shortening and the diffracting action of single, functionally intact, unattached cardiac muscle cells enzymatically isolated from the ventricular tissue of adult rats. Sarcomere length was measured either (a) continuously by a light diffraction method or (b) by direct inspection of the cell's striated image as recorded on videotape or by cinemicroscopy (120-400 frames/s). At physiological levels of added CaCl2 (0.5- 2.0 mM), many cells were quiescent (i.e., they did not beat spontaneously) and contracted in response to electrical stimulation (~ 1.0-ms pulse width). Sarcomere length in the quiescent, unstimulated cells (1.93 + 0.10 [SD] /~m), at peak shortening (1.57 + 0.13/~m, n = 49), and the maximum velocity of sarcomere shortening and relengthening were comparable to previous observations in intact heart muscle preparations. The dispersion of light diffracted by the cell remained narrow, and individual striations remained distinct and laterally well registered throughout the shortening-relengthening cycle. In contrast, apprecia- ble nonuniformity and internal buckling were seen at sarcomere lengths < 1.8 /~m when the resting cell, embedded in gelatin, was longitudinally compressed. These results indicate (a) that shortening and relengthening is characterized by uniform activation between myofibrils within the cardiac cell and (b) that physiologically significant relengthening forces in living heart muscle originate at the level of the cell rather than in extracellular connections. -

Titin N2A Domain and Its Interactions at the Sarcomere
International Journal of Molecular Sciences Review Titin N2A Domain and Its Interactions at the Sarcomere Adeleye O. Adewale and Young-Hoon Ahn * Department of Chemistry, Wayne State University, Detroit, MI 48202, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-(313)-577-1384 Abstract: Titin is a giant protein in the sarcomere that plays an essential role in muscle contraction with actin and myosin filaments. However, its utility goes beyond mechanical functions, extending to versatile and complex roles in sarcomere organization and maintenance, passive force, mechanosens- ing, and signaling. Titin’s multiple functions are in part attributed to its large size and modular structures that interact with a myriad of protein partners. Among titin’s domains, the N2A element is one of titin’s unique segments that contributes to titin’s functions in compliance, contraction, structural stability, and signaling via protein–protein interactions with actin filament, chaperones, stress-sensing proteins, and proteases. Considering the significance of N2A, this review highlights structural conformations of N2A, its predisposition for protein–protein interactions, and its multiple interacting protein partners that allow the modulation of titin’s biological effects. Lastly, the nature of N2A for interactions with chaperones and proteases is included, presenting it as an important node that impacts titin’s structural and functional integrity. Keywords: titin; N2A domain; protein–protein interaction 1. Introduction Citation: Adewale, A.O.; Ahn, Y.-H. The complexity of striated muscle is defined by the intricate organization of its com- Titin N2A Domain and Its ponents [1]. The involuntary cardiac and voluntary skeletal muscles are the primary types Interactions at the Sarcomere. -

Breakdown of Filamentous Myofibrils by the UPS–Step by Step
biomolecules Review Breakdown of Filamentous Myofibrils by the UPS–Step by Step Dina Aweida and Shenhav Cohen * Faculty of Biology, Technion Institute of Technology, Haifa 32000, Israel; [email protected] * Correspondence: [email protected]; Tel.: +972-4-8294214 Abstract: Protein degradation maintains cellular integrity by regulating virtually all biological pro- cesses, whereas impaired proteolysis perturbs protein quality control, and often leads to human disease. Two major proteolytic systems are responsible for protein breakdown in all cells: autophagy, which facilitates the loss of organelles, protein aggregates, and cell surface proteins; and the ubiquitin- proteasome system (UPS), which promotes degradation of mainly soluble proteins. Recent findings indicate that more complex protein structures, such as filamentous assemblies, which are not acces- sible to the catalytic core of the proteasome in vitro, can be efficiently degraded by this proteolytic machinery in systemic catabolic states in vivo. Mechanisms that loosen the filamentous structure seem to be activated first, hence increasing the accessibility of protein constituents to the UPS. In this review, we will discuss the mechanisms underlying the disassembly and loss of the intricate insoluble filamentous myofibrils, which are responsible for muscle contraction, and whose degradation by the UPS causes weakness and disability in aging and disease. Several lines of evidence indicate that myofibril breakdown occurs in a strictly ordered and controlled manner, and the function of AAA-ATPases is crucial for their disassembly and loss. Keywords: ubiquitin; proteasome; autophagy; muscle atrophy; intermediate filaments; myofibrils; AAA-ATPases 1. Introduction Citation: Aweida, D.; Cohen, S. Proteolysis promotes tissue homeostasis by controlling protein abundance in response Breakdown of Filamentous Myofibrils to extracellular and intracellular cues, and by preventing accumulation of misfolded or by the UPS–Step by Step. -

Effect of Camkiiδ Deletion on Sarcomere Structure
Features Efect of CaMKIIδ Deletion on Sarcomere Structure Tik-Chee (Jenny) Cheng, Sukriti Dewan, Andrew McCulloch, Jef Omens Jenny Cheng is a senior at Earl Warren College majoring in Bioengineering with a focus in Biotechnology. In Fall 2015, she will be pursuing her PhD in Biomedical Engineering at the University of Wisconsin-Madison, studying cardiovascular pathophysiology for clinical applications. Heart failure, a progressive cardiomyopathy, a condition with condition that is typically enlarged left ventricles and/or undetected and often dilated ventricular walls that can misdiagnosed, is the leading lead to dysfunction and inadequate cause of deaths around pumping of blood to the rest of the world. The disorder is the body. Calcium/calmodulin- CaMKII isoform (CaMKIIδ) characterized by the inability dependent protein kinase II appear to be protected of the heart to keep up with (CaMKII) is a protein kinase from such dysfunction and the body’s demand for blood that can phosphorylate many hypertrophy. circulation. It is initiated substrates and regulates a wide by structural and functional range of cellular function. Its role Normal myocardial function changes from in calcium signaling is dependent on the structure The disorder is molecular to pathways has been of cardiomyocytes, specifcally systemic levels characterized by implicated in the the basic subunits of muscle that result in the inability of the pathophysiology called sarcomeres. Sarcomeres compensatory cardiac pump to of adverse cardiac contract and generate force physiological keep up with the left ventricular by utilizing the motor changes, body’s demand. remodeling. movements of thin and thick commonly Animal models flaments, specifcally myosin termed cardiac remodeling. -

Defects in T-Tubular Electrical Activity Underlie Local Alterations of Calcium Release in Heart Failure
Defects in T-tubular electrical activity underlie local alterations of calcium release in heart failure Claudia Crocinia, Raffaele Coppinib, Cecilia Ferrantinic, Ping Yand, Leslie M. Loewd, Chiara Tesic, Elisabetta Cerbaib, Corrado Poggesic, Francesco S. Pavonea,e,f, and Leonardo Sacconia,f,1 aEuropean Laboratory for Non-Linear Spectroscopy, 50019 Florence, Italy; bDivision of Pharmacology, Department “NeuroFarBa,” University of Florence, 50139 Florence, Italy; cDivision of Physiology, Department of Experimental and Clinical Medicine, University of Florence, 50134 Florence, Italy; dR. D. Berlin Center for Cell Analysis and Modeling, University of Connecticut Health Center, Farmington, CT 06030; eDepartment of Physics and Astronomy, University of Florence, 50019 Sesto Fiorentino, Italy; and fNational Institute of Optics, National Research Council, 50125 Florence, Italy Edited by Clara Franzini-Armstrong, University of Pennsylvania Medical Center, Philadelphia, PA, and approved September 15, 2014 (received for review June 20, 2014) Action potentials (APs), via the transverse axial tubular system in a rat model of postischemic HF, structurally remodeled TATS + (TATS), synchronously trigger uniform Ca2 release throughout the exhibits abnormal electrical activity, i.e., failure of AP propagation cardiomyocyte. In heart failure (HF), TATS structural remodeling and presence of local spontaneous depolarizations. Tubular AP occurs, leading to asynchronous Ca2+ release across the myocyte failures and spontaneous activity can potentially aggravate asyn- + and contributing to contractile dysfunction. In cardiomyocytes from chronous Ca2 release and determine nonhomogeneous myofibril + failing rat hearts, we previously documented the presence of TATS contraction. Simultaneous recording of local Ca2 release and AP in elements which failed to propagate AP and displayed spontaneous the tubular network is needed to unravel the consequences of these + + electrical activity; the consequence for Ca2 release remained, how- electrical anomalies on intracellular Ca2 dynamics.