Air Pollution and Adverse Health Effects: Assessing Exposure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Educated Youth Should Go to the Rural Areas: a Tale of Education, Employment and Social Values*
Educated Youth Should Go to the Rural Areas: A Tale of Education, Employment and Social Values* Yang You† Harvard University This draft: July 2018 Abstract I use a quasi-random urban-dweller allocation in rural areas during Mao’s Mass Rustication Movement to identify human capital externalities in education, employment, and social values. First, rural residents acquired an additional 0.1-0.2 years of education from a 1% increase in the density of sent-down youth measured by the number of sent-down youth in 1969 over the population size in 1982. Second, as economic outcomes, people educated during the rustication period suffered from less non-agricultural employment in 1990. Conversely, in 2000, they enjoyed increased hiring in all non-agricultural occupations and lower unemployment. Third, sent-down youth changed the social values of rural residents who reported higher levels of trust, enhanced subjective well-being, altered trust from traditional Chinese medicine to Western medicine, and shifted job attitudes from objective cognitive assessments to affective job satisfaction. To explore the mechanism, I document that sent-down youth served as rural teachers with two new county-level datasets. Keywords: Human Capital Externality, Sent-down Youth, Rural Educational Development, Employment Dynamics, Social Values, Culture JEL: A13, N95, O15, I31, I25, I26 * This paper was previously titled and circulated, “Does living near urban dwellers make you smarter” in 2017 and “The golden era of Chinese rural education: evidence from Mao’s Mass Rustication Movement 1968-1980” in 2015. I am grateful to Richard Freeman, Edward Glaeser, Claudia Goldin, Wei Huang, Lawrence Katz, Lingsheng Meng, Nathan Nunn, Min Ouyang, Andrei Shleifer, and participants at the Harvard Economic History Lunch Seminar, Harvard Development Economics Lunch Seminar, and Harvard China Economy Seminar, for their helpful comments. -

Regional Ecological Risk Assessment of Wetlands in the Sanjiang Plain with Respect to Human Disturbance
sustainability Article Regional Ecological Risk Assessment of Wetlands in the Sanjiang Plain with Respect to Human Disturbance Hui Wang 1,2, Changchun Song 2,* and Kaishan Song 2 1 College of Tourism and Geography, Jiujiang University, Jiujiang 332005, China; [email protected] 2 Key Laboratory of Wetland Ecology and Environment, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun 130102, China; [email protected] * Correspondence: [email protected] Received: 31 December 2019; Accepted: 27 February 2020; Published: 5 March 2020 Abstract: Characterization of the intensity of regional human disturbances on wetlands is an important scientific issue. In this study, the pole-axis system (involving multi-level central places and roads) was recognized as a proxy of direct risk to wetlands stemming from human activities at the regional or watershed scale. In this respect, the pole-axis system and central place theory were adopted to analyze the spatial agglomeration characteristics of regional human activities. Soil erosion and non-point source (NPS) pollution, indicating the indirect effect of human activities on wetlands, were also considered. Based on these human disturbance proxies, which are considered regional risk sources to wetlands, incorporated with another two indicators of regional environment, i.e., vulnerability and ecological capital indexes, the regional ecological risk assessment (RERA) framework of wetlands was finally established. Using this wetland RERA framework, the spatial heterogeneity -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

Spatiotemporal Changes of Reference Evapotranspiration in the Highest-Latitude Region of China
Article Spatiotemporal Changes of Reference Evapotranspiration in the Highest-Latitude Region of China Peng Qi 1,2, Guangxin Zhang 1,*, Y. Jun Xu 3, Yanfeng Wu 1,2 and Zongting Gao 4 1 Key Laboratory of Wetland Ecology and Environment, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, No. 4888, Shengbei Street, Changchun 130102, China; [email protected] (P.Q.); [email protected] (Y.W.) 2 University of the Chinese Academy of Sciences, Beijing 100049, China 3 School of Renewable Natural Resources, Louisiana State University Agricultural Center, Baton Rouge, LA 70803, USA; [email protected] 4 Institute of Meteorological Sciences of Jilin Province, and Laboratory of Research for Middle-High Latitude Circulation and East Asian Monsoon, and Jilin Province Key Laboratory for Changbai Mountain Meteorology and Climate Change, Changchun 130062, China; [email protected] * Correspondence: [email protected]; Tel.: +86-431-8554-2210; Fax: +86-431-8554-2298 Received: 2 June 2017; Accepted: 4 July 2017; Published: 5 July 2017 Abstract: Reference evapotranspiration (ET0) is often used to make management decisions for crop irrigation scheduling and production. In this study, the spatial and temporal trends of ET0 in China’s most northern province as well as the country’s largest agricultural region were analyzed for the period from 1964 to 2013. ET0 was calculated with the Penman-Monteith of Food and Agriculture Organization of the United Nations irrigation and drainage paper NO.56 (FAO-56) using climatic data collected from 27 stations. Inverse distance weighting (IDW) was used for the spatial interpolation of the estimated ET0. A Modified Mann–Kendall test (MMK) was applied to test the spatiotemporal trends of ET0, while Pearson’s correlation coefficient and cross-wavelet analysis were employed to assess the factors affecting the spatiotemporal variability at different elevations. -

The Impact of Continuous Driving Time and Rest Time on Commercial Drivers' Driving Performance and Recovery
Journal of Safety Research 50 (2014) 11–15 Contents lists available at ScienceDirect Journal of Safety Research journal homepage: www.elsevier.com/locate/jsr The impact of continuous driving time and rest time on commercial drivers' driving performance and recovery Lianzhen Wang a,⁎,YulongPeib a School of Transportation Science and Engineering, Harbin Institute of Technology, Harbin 150090, China b Traffic College, Northeast Forestry University, Harbin 150040, China article info abstract Article history: Problem: This real road driving study was conducted to investigate the effects of driving time and rest time on the Received 16 March 2013 driving performance and recovery of commercial coach drivers. Methods: Thirty-three commercial coach drivers Received in revised form 11 January 2014 participated in the study, and were divided into three groups according to driving time: (a) 2 h, (b) 3 h, and (c) 4 Accepted 15 January 2014 h. The Stanford Sleepiness Scale (SSS) was used to assess the subjective fatigue level of the drivers. One-way Available online 28 January 2014 ANOVA was employed to analyze the variation in driving performance. Results: The statistical analysis revealed that driving time had a significant effect on the subjective fatigue and driving performance measures among Keywords: the three groups. After 2 h of driving, both the subjective fatigue and driving performance measures began to de- Fatigue driving fi Driving performance teriorate. After 4 h of driving, all of the driving performance indicators changed signi cantly except for depth per- Commercial coach drivers ception. A certain amount of rest time eliminated the negative effects of fatigue. A 15-minute rest allowed drivers Driving time to recover from a two-hour driving task. -
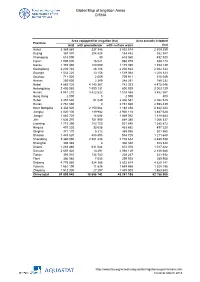
Global Map of Irrigation Areas CHINA
Global Map of Irrigation Areas CHINA Area equipped for irrigation (ha) Area actually irrigated Province total with groundwater with surface water (ha) Anhui 3 369 860 337 346 3 032 514 2 309 259 Beijing 367 870 204 428 163 442 352 387 Chongqing 618 090 30 618 060 432 520 Fujian 1 005 000 16 021 988 979 938 174 Gansu 1 355 480 180 090 1 175 390 1 153 139 Guangdong 2 230 740 28 106 2 202 634 2 042 344 Guangxi 1 532 220 13 156 1 519 064 1 208 323 Guizhou 711 920 2 009 709 911 515 049 Hainan 250 600 2 349 248 251 189 232 Hebei 4 885 720 4 143 367 742 353 4 475 046 Heilongjiang 2 400 060 1 599 131 800 929 2 003 129 Henan 4 941 210 3 422 622 1 518 588 3 862 567 Hong Kong 2 000 0 2 000 800 Hubei 2 457 630 51 049 2 406 581 2 082 525 Hunan 2 761 660 0 2 761 660 2 598 439 Inner Mongolia 3 332 520 2 150 064 1 182 456 2 842 223 Jiangsu 4 020 100 119 982 3 900 118 3 487 628 Jiangxi 1 883 720 14 688 1 869 032 1 818 684 Jilin 1 636 370 751 990 884 380 1 066 337 Liaoning 1 715 390 783 750 931 640 1 385 872 Ningxia 497 220 33 538 463 682 497 220 Qinghai 371 170 5 212 365 958 301 560 Shaanxi 1 443 620 488 895 954 725 1 211 648 Shandong 5 360 090 2 581 448 2 778 642 4 485 538 Shanghai 308 340 0 308 340 308 340 Shanxi 1 283 460 611 084 672 376 1 017 422 Sichuan 2 607 420 13 291 2 594 129 2 140 680 Tianjin 393 010 134 743 258 267 321 932 Tibet 306 980 7 055 299 925 289 908 Xinjiang 4 776 980 924 366 3 852 614 4 629 141 Yunnan 1 561 190 11 635 1 549 555 1 328 186 Zhejiang 1 512 300 27 297 1 485 003 1 463 653 China total 61 899 940 18 658 742 43 241 198 52 -

Heilongjiang Road Development II Project (Yichun-Nenjiang)
Technical Assistance Consultant’s Report Project Number: TA 7117 – PRC October 2009 People’s Republic of China: Heilongjiang Road Development II Project (Yichun-Nenjiang) FINAL REPORT (Volume II of IV) Submitted by: H & J, INC. Beijing International Center, Tower 3, Suite 1707, Beijing 100026 US Headquarters: 6265 Sheridan Drive, Suite 212, Buffalo, NY 14221 In association with WINLOT No 11 An Wai Avenue, Huafu Garden B-503, Beijing 100011 This consultant’s report does not necessarily reflect the views of ADB or the Government concerned, ADB and the Government cannot be held liable for its contents. All views expressed herein may not be incorporated into the proposed project’s design. Asian Development Bank Heilongjiang Road Development II (TA 7117 – PRC) Final Report Supplementary Appendix A Financial Analysis and Projections_SF1 S App A - 1 Heilongjiang Road Development II (TA 7117 – PRC) Final Report SUPPLEMENTARY APPENDIX SF1 FINANCIAL ANALYSIS AND PROJECTIONS A. Introduction 1. Financial projections and analysis have been prepared in accordance with the 2005 edition of the Guidelines for the Financial Governance and Management of Investment Projects Financed by the Asian Development Bank. The Guidelines cover both revenue earning and non revenue earning projects. Project roads include expressways, Class I and Class II roads. All will be built by the Heilongjiang Provincial Communications Department (HPCD). When the project started it was assumed that all project roads would be revenue earning. It was then discovered that national guidance was that Class 2 roads should be toll free. The ADB agreed that the DFR should concentrate on the revenue earning Expressway and Class I roads, 2. -

Preparing the Heilongjiang Road Development II Project (Yichun–Nenjiang)
Technical Assistance Report Project Number: 42017 August 2008 People’s Republic of China: Preparing the Heilongjiang Road Development II Project (Yichun–Nenjiang) The views expressed herein are those of the consultant and do not necessarily represent those of ADB’s members, Board of Directors, Management, or staff, and may be preliminary in nature. CURRENCY EQUIVALENTS (as of 1 July 2008) Currency Unit – yuan (CNY) CNY1.00 = $0.1459 $1.00 = CNY6.8543 ABBREVIATIONS ADB – Asian Development Bank C&P – consultation and participatory EIA – environmental impact assessment GDP – gross domestic product HPCD – Heilongjiang Provincial Communications Department IPSA – initial poverty and social analysis PRC – People’s Republic of China TA – technical assistance TECHNICAL ASSISTANCE CLASSIFICATION Targeting Classification – General intervention Sector – Transport and communications Subsector – Roads and highways Themes – Sustainable economic growth, capacity development Subthemes – Fostering physical infrastructure development, institutional development NOTE In this report, "$" refers to US dollars. Vice-President C. Lawrence Greenwood, Jr., Operations 2 Director General K. Gerhaeusser, East Asia Department (EARD) Director C.S. Chin, Officer-in-Charge, Transport Division, EARD Team leader E. Oyunchimeg, Transport Specialist (Roads), EARD Team members S. Ferguson, Senior Social Development Specialist (Resettlement), EARD T. Yokota, Transport Specialist, EARD 121o 00'E 132o 00'E HEILONGJIANG ROAD DEVELOPMENT II PROJECT (YICHUN--NENJIANG) IN THE PEOPLE'S REPUBLIC OF CHINA RUSSIAN FEDERATION Provincial Capital City/Town Mohe County Highway Port National Key Tourism Scenic Spot H eilong River Proposed Project Road Tahe ADB--Financed Road Loop Line Radial Line Huma Vertical Line Horizontal Line River Provincial Boundary 52 o 00'N 52 o 00'N International Boundary Woduhe Jiagdaqi Boundaries are not necessarily authoritative. -

Download 448.33 KB
Environmental Assessment Report Summary Environmental Impact Assessment Project Number: 39038 February 2006 People’s Republic of China: Heilongjiang Road Network Development Project Prepared by Heilongjiang Provincial Communications Department for the Asian Development Bank (ADB). The The summaryviews expressed environmental herein areimpact those assessment of the consul is tanta document and do not of necessarilythe borrower. represent The views those expressed of ADB’s herein members, do not Board necessarily of Directors, represent Management, those of orADB’s staff, Boardand may of Directors,be preliminary Mana ingement, nature. or staff, and may be preliminary in nature. CURRENCY EQUIVALENTS (as of 13 February 2006) Currency Unit – yuan (CNY) CNY1.00 = $0.1242 $1.00 = CNY8.0505 ABBREVIATIONS ADB – Asian Development Bank BOD5 – biological oxygen demand (5-day) CO – carbon monoxide COD – chemical oxygen demand EIA – environmental impact assessment EMP – environmental management plan GB – guojia biaozhun (national standard) HIV/AIDS – human immunodeficiency syndrome/acquired immunodeficiency syndrome HPCD – Heilongjiang Provincial Communications Department NO2 – nitrogen dioxide PRC – People’s Republic of China RP – resettlement plan SEIA – summary environmental impact assessment SEPP – soil erosion prevention plan TSP – total suspended particles WEIGHTS AND MEASURES dB(A) – decibel (measured in audible noise bands) ha – hectare km – kilometer km2 – square kilometer m – meter m2 – square meter m3 – cubic meter mg – milligram MTE – medium truck equivalent mu – unit of area commonly used in the PRC; 15 mu = 1 hectare NOTE In this report, "$" refers to US dollars. CONTENTS Page MAP I. INTRODUCTION 1 II. DESCRIPTION OF THE PROJECT 1 III. DESCRIPTION OF THE ENVIRONMENT 2 A. Physical Resources 2 B. -

Heilongjiang Green Urban and Economic Revitalization Project
Draft Environmental Impact Assessment May 2017 People’s Republic of China: Heilongjiang Green Urban and Economic Revitalization Project Part 3 Prepared by the Heilongjiang Provincial Government for the Asian Development Bank. 293. JX 4.3 Gonggu Flyover: air quality. Table VI-24 presents the predicted maximum pollutant concentrations near the Gonggu Flyover boundary, with modeling results shown in Figure VI-13. The results indicate that the maximum CO and NO2 ground-level concentration values will comply with Class II standard limits of Ambient Air Quality Standard (GB 3095-2012) during future operation. Table VI-24: Maximum pollutant concentration projections for Gongqu Flyover (Jixi) Sensitive Pollutant Year Predicted value Contribution mg Baseline mg/m3 Prediction Standard Receptor mg /m3 /m3 mg/m3 mg/m3 Baoquanwei NO2 Short term 1h 0.0162 0.02024 0.062 0.08224 0.2 (2022) 24h 0.0162 0.00452 0.055 0.05952 0.08 Annual 0.00163 0.00163 0.04 Medium term 1h 0.0207 0.02450 0.062 0.08650 0.2 (2027) 24h 0.0207 0.00547 0.055 0.06047 0.08 Annual 0.00197 0.00197 0.04 Long term 1h 0.0256 0.03160 0.062 0.09360 0.2 (2037) 24h 0.0256 0.00705 0.055 0.06205 0.08 Annual 0.00254 0.00254 0.04 6.36 CO Short term 1h 0.1015 0.12461 1.2 1.32461 10 (2022) 24h 0.1015 0.02780 1.1 1.12780 4 Medium term 1h 0.1275 0.15088 1.2 1.35088 10 (2027) 24h 0.1275 0.03367 1.1 1.13367 4 Long term 1h 0.1579 0.19525 1.2 1.39525 10 (2037) 24h 0.1579 0.04357 1.1 1.14357 4 Maximum NO2 Short term 1h 0.0162 0.04695 0.062 0.10895 0.2 ground (2022) 24h 0.0162 0.00854 0.055 0.06354 0.08 -

"Everydays" (A Progression Inspired by Jerome Kern) Schoenhals, Michael
"Everydays" (A Progression Inspired by Jerome Kern) Schoenhals, Michael 2013 Link to publication Citation for published version (APA): Schoenhals, M. (2013). "Everydays" (A Progression Inspired by Jerome Kern). V:1-V:11. Paper presented at 2013 WCU Alltagsgeschichte Transnational Workshop: Politics of Memory, Practices of Remembrance, . Total number of authors: 1 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Prepared for the Alltagswoche Fest “Politics of Memory, Practices of Remebrances,” Hanyang University, Seoul, April 17–20, 2013. Please do not quote! “Everydays” (A Progression Inspired by Jerome Kern) MICHAEL SCHOENHALS Lund University Shortly before Christmas 2012, in a Beijing flea market and at a price, I was offered a cache of more than two thousand moth-eaten surveillance (“stake-out”) logs from the early 1950s emanating with the 1st Section of the municipal Bureau of Public Security in Harbin, Manchuria. -

Comparative Genomics and Association Analysis Identifies
Gu et al. BMC Genomics (2020) 21:172 https://doi.org/10.1186/s12864-020-6581-5 RESEARCH ARTICLE Open Access Comparative genomics and association analysis identifies virulence genes of Cercospora sojina in soybean Xin Gu1,2, Junjie Ding2, Wei Liu2, Xiaohe Yang2, Liangliang Yao2, Xuedong Gao2, Maoming Zhang2, Shuai Yang3 and Jingzhi Wen1* Abstract Background: Recently, a new strain of Cercospora sojina (Race15) has been identified, which has caused the breakdown of resistance in most soybean cultivars in China. Despite this serious yield reduction, little is known about why this strain is more virulent than others. Therefore, we sequenced the Race15 genome and compared it to the Race1 genome sequence, as its virulence is significantly lower. We then re-sequenced 30 isolates of C. sojina from different regions to identifying differential virulence genes using genome-wide association analysis (GWAS). Results: The 40.12-Mb Race15 genome encodes 12,607 predicated genes and contains large numbers of gene clusters that have annotations in 11 different common databases. Comparative genomics revealed that although these two genomes had a large number of homologous genes, their genome structures have evolved to introduce 245 specific genes. The most important 5 candidate virulence genes were located on Contig 3 and Contig 1 and were mainly related to the regulation of metabolic mechanisms and the biosynthesis of bioactive metabolites, thereby putatively affecting fungi self-toxicity and reducing host resistance. Our study provides insight into the genomic basis of C. sojina pathogenicity and its infection mechanism, enabling future studies of this disease. Conclusions: Via GWAS, we identified five candidate genes using three different methods, and these candidate genes are speculated to be related to metabolic mechanisms and the biosynthesis of bioactive metabolites.