Circulatory Changes at Birth [1]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power
DSJUOG Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology REVIEW ARTICLE Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology 1Ritsuko K Pooh, 2Asim Kurjak 1CRIFM Clinical Research Institute of Fetal Medicine PMC, Osaka, Japan 2Department of Obstetrics and Gynecology, University of Zagreb, School of Medicine, Zagreb, Croatia Correspondence: Ritsuko K Pooh, CRIFM Clinical Research Institute of Fetal Medicine PMC 7-3-7, Uehommachi, Tennoji Osaka #543-0001, Japan, Phone: +81-6-6775-8111, Fax: +81-6-6775-8122, e-mail: [email protected] Abstract Significant advances have been made in accurate and reliable visualization of the cerebral circulation in normal and abnormal pregnancies. They provided the non-invasive studies of fetal cerebral angiogenesis and further development that filled some of the gaps made by neuroanatomical studies alone. The first breakthrough in the assessment of fetal circulation was development of Doppler system with purpose to obtain velocity waveforms. Continuing technical advances in Doppler ultrasound equipment, especially highly sensitive color flow imagining techniques have made it possible to study smaller anatomical parts of fetal circulation system including cerebral vascularization. Before examination of brain vascularity, anatomical vascular structure and development on the different appearance at each gestational age should be remembered as the basic knowledge. Since the development of the embryo is rapid and significant changes occur during even one week it is important to specify the stage of the embryo or fetus both by age (postmenstrual weeks and days) and by size (crownrump length (CRL) and biparietal diameter (BPD). Introduction of three-dimensional (3D) sonography and 3D power Doppler techniques have enabled visualization of intracranial vessels. -
Development of Right Ventricle
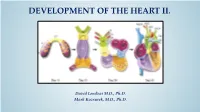
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

The Levels of Angiogenic and Anti-Angiogenic Molecule Concentrations in Pregnancy Based Disorders in the Maternal and Fetal Circ
Master of Philosophy THE LEVELS OF ANGIOGENIC AND ANTI-ANGIOGENIC MOLECULE CONCENTRATIONS IN PREGNANCY BASED DISORDERS IN THE MATERNAL AND FETAL CIRCULATION Islam Afzal Supervisor: Professor Asif Ahmed Department of Reproductive and Vascular Biology November 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Islam Afzal Contents Table of Figures ............................................................................................... 5 Abbreviations ................................................................................................... 7 Acknowledgements .......................................................................................... 9 Abstract .......................................................................................................... 10 Introduction .................................................................................................... 11 Preeclampsia ................................................................................................ -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Equine Placenta – Marvelous Organ and a Lethal Weapon
Equine placenta – marvelous organ and a lethal weapon Malgorzata Pozor, DVM, PhD, Diplomate ACT Introduction Placenta has been defined as: „an apposition between parent (usually maternal) and fetal tissue in order to establish physiological exchange” (1). Another definition of this important organ was proposed by Steven and Morris: „a device consisting of one or more transport epithelia located between fetal and maternal blood supply” (2). The main function of placenta is to provide an interface between the dam and the the fetus and to allow the metabolic exchange of the the nutrients, oxygen and waste material. The maternal circulation is brought into a close apposition to the fetal circulation, while a separation of these two circulatory systems remain separated (3). A degree and complexity of this „intimate relationship” varies greately between species mostly due to the structural diversity of the extraembryonic membranes of the vertebrates. The early feto-maternal exchange in the equine pregnancy is established as early as on day 22 after fertilization. The fetal and choriovitellin circulations are already present, the capsule ruptures and the allantois is already visible (4). The allantois starts expanding by day 32 and vascularizes approximately 90% of the chorion and fuses with it to form chorioallantois by day 38 of gestation (5). The equine placenta continues increasing its complexity till approximately day 150 of gestation. Equids have epitheliochorial placenta, there are six leyers separating maternal and fetal circulation, and there are no erosion of the luminal, maternal epithelium, like in ruminants (6). Thousands of small chorionic microvilli develop and penetrate into endometrial invaginations. -

From Trophoblast to Human Placenta
From Trophoblast to Human Placenta (from The Encyclopedia of Reproduction) Harvey J. Kliman, M.D., Ph.D. Yale University School of Medicine I. Introduction II. Formation of the placenta III. Structure and function of the placenta IV. Complications of pregnancy related to trophoblasts and the placenta Glossary amnion the inner layer of the external membranes in direct contact with the amnionic fluid. chorion the outer layer of the external membranes composed of trophoblasts and extracellular matrix in direct contact with the uterus. chorionic plate the connective tissue that separates the amnionic fluid from the maternal blood on the fetal surface of the placenta. chorionic villous the final ramification of the fetal circulation within the placenta. cytotrophoblast a mononuclear cell which is the precursor cell of all other trophoblasts. decidua the transformed endometrium of pregnancy intervillous space the space in between the chorionic villi where the maternal blood circulates within the placenta invasive trophoblast the population of trophoblasts that leave the placenta, infiltrates the endo– and myometrium and penetrates the maternal spiral arteries, transforming them into low capacitance blood channels. Sunday, October 29, 2006 Page 1 of 19 From Trophoblasts to Human Placenta Harvey Kliman junctional trophoblast the specialized trophoblast that keep the placenta and external membranes attached to the uterus. spiral arteries the maternal arteries that travel through the myo– and endometrium which deliver blood to the placenta. syncytiotrophoblast the multinucleated trophoblast that forms the outer layer of the chorionic villi responsible for nutrient exchange and hormone production. I. Introduction The precursor cells of the human placenta—the trophoblasts—first appear four days after fertilization as the outer layer of cells of the blastocyst. -

Cardiovascular System - Accessscience from Mcgraw-Hill Education
Cardiovascular system - AccessScience from McGraw-Hill Education http://accessscience.com/content/109900 (http://accessscience.com/) Article by: Weichert, Charles K. College of Arts and Sciences, University of Cincinnati, Cincinnati, Ohio. Copenhaver, W. M. College of Physicians and Surgeons, Columbia University, New York; Department of Biological Structures, School of Medicine, University of Miami, Miami, Florida. Ebert, James D. Department of Embryology, Carnegie Institution, Washington, DC. Patten, Bradley M. Department of Anatomy, University of Michigan, Ann Arbor, Michigan. Jones, David R. Department of Zoology, University of British Columbia, Vancouver, Canada. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.109900 (http://dx.doi.org/10.1036/1097-8542.109900) Content Comparative Anatomy Embryogenesis of blood vessels Balancing ventricular output Heart Angiogenesis Human Postnatal Circulation Arterial system Circulatory system morphogenesis Pulmonary circuit and ductus Venous system Primitive venous system Physiological aspects of transition Comparative Embryology Functional Development of Heart Comparative Physiology Heart Contractions of the heart General physiology of circulation Tubular heart formation Heart-forming areas Microcirculation Cardiac loop and regional development Contractile proteins Heart Formation of definitive heart Synthesis of contractile proteins Arteries Partitioning of mammalian heart Action of inhibitors Venous system Division of atrium and ventricles Human Fetal Circulation at Term Bibliography -

5. Development of Circulatory System I. Embryonic and Extraembryonic Circulation
Z. Tonar, M. Králíčková: Outlines of lectures on embryology for 2 nd year student of General medicine and Dentistry License Creative Commons - http://creativecommons.org/licenses/by-nc-nd/3.0/ 5. Development of circulatory system I. Embryonic and extraembryonic circulation. Aortic arches. Veins. Blood vessels − vasculogenesis = vessels arise from mesenchymal blood islands o in week 3, cells named angioblasts condense and form blood islands within extraembryonic mesenchyme in the wall of yolk sac, connecting stalk, and chorion o in the embryo, cells migrate mainly from the intraembryonic mesoderm to form undifferentiated embryonáic mesenchyme; in this mesenchyme, angioblasts condense into blood islands as well o the blood islands luminize, thus becoming primitive vascular canals lined with endothelial cells and containing primitive blood cells named erytroblasts − angiogenesise = vessels sprout from existing vessels Early bilateral circulation − umbilical veins: these carry oxygenated blood from the chorionic villi via the connecting stalk to the embryo into the heart tube − umbilical arteries: these carry blood from the dorsal aorta towards the chorionic villi − vitelline artery: from the dorsal aorta towards the extraembryonic vitelline circulation within the wall of yolk sac − vitelline vein: from the vitelline circulation of the yolk sac towards the heart − segmental arteries arise from the dorsal aorta and pass between the body somites − the venous drainage from the somites is collected via segmental veins which fuse into the anterior -

Lymphspiration Are There Lymphatic Vessels in the Placenta?
34 Lymphology 45 (2012) 34-36 LYMPHSPIRATION ARE THERE LYMPHATIC VESSELS IN THE PLACENTA? C. Bellini, M. Rutigliani, F. Boccardo, C. Campisi, T. Bellini, E. Bonioli, E. Fulcheri Neonatal Intensive Care Unit (CB), Department of Pediatrics (TB, EB), University of Genoa, I.R.C.C.S. Gaslini Institute, Genoa; Department of Pathology (MR), I.R.C.C.S. Maugeri Foundation, Pavia, Italy; Department of Surgery (FB, CC), Unit of Lymphatic Surgery, S. Martino Hospital, University of Genoa, Genoa; and Department of Pathology (EF), University of Genoa, Genoa, Italy ABSTRACT tolerance of the semi-allogeneic fetus and fluid balance between maternal and fetal The role of lymphatics in placentation compartments (1) are fundamental biological has been scantily studied and the true processes linked to placental functions. The existence of placental lymphatics is under lymphatics play a role in both of these func- debate. Numerous blood and lymphatic- tions. Although these important placental lineage molecule markers are now available functions have been under investigation for a and they are expressed in human placental long time, the role of lymphatics in placenta- tissue. D2-40 expression at the placental tion has been scantily studied and the true stromal level seems to indicate that network- existence of placental lymphatics is under forming, podoplanin-expressing cells may act debate. Furthermore, the few available data as a reticular-lymphatic-like conductive are contradictory. network. This exciting area at the intersection Thus, the main query might be: are of perinatology and lymphology needs further there lymphatics in the human placenta? investigation. And, moreover, how can we approach this problem? Keywords: placenta; lymphatics; During pregnancy, utero-placental blood immunohistochemistry, D2-40 podoplanin flow increases progressively, with estimates ranging from 600-700 ml/min near term. -

The Fetal Circulation
PRENATAL DIAGNOSIS Prenat Diagn 2004; 24: 1049–1059. Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/pd.1062 REVIEW The fetal circulation Torvid Kiserud1* and Ganesh Acharya2 1University of Bergen, Department of Obstetrics and Gynecology, Bergen, Norway 2Department of Obstetrics and Gynecology, University Hospital of Northern Norway, Tromsø, Norway Accumulating data on the human fetal circulation shows the similarity to the experimental animal physiology, but with important differences. The human fetus seems to circulate less blood through the placenta, shunt less through the ductus venosus and foramen ovale, but direct more blood through the lungs than the fetal sheep. However, there are substantial individual variations and the pattern changes with gestational age. The normalised umbilical blood flow decreases with gestational age, and, at 28 to 32 weeks, a new level of development seems to be reached. At this stage, the shunting through the ductus venosus and the foramen ovale reaches a minimum, and the flow through the lungs a maximum. The ductus venosus and foramen ovale are functionally closely related and represent an important distributional unit for the venous return. The left portal branch represents a venous watershed, and, similarly, the isthmus aorta an arterial watershed. Thus, the fetal central circulation is a very flexible and adaptive circulatory system. The responses to increased afterload, hypoxaemia and acidaemia in the human fetus are equivalent to those found in animal studies: increased ductus venosus and foramen ovale shunting, increased impedance in the lungs, reduced impedance in the brain, increasingly reversed flow in the aortic isthmus and a more prominent coronary blood flow. -

Faculty of Nursing Fetal Circulation
FACULTY OF NURSING FETAL CIRCULATION Mrs.Jasmi Manu Asso.professor cum head of the department (OBS/GYN) Faculty of Nursing ,Rama University,kanpur INTRODUCTION In the fully developed human, the heart serves two main purposes. The right heart pumps blood to the lungs for oxygenation and the left heart pumps oxygenated blood to rest of the body. In the embryo and fetus, the lungs do not oxygenate the blood. Fetal circulation is consequently quite different than that of a breathing baby or adult. When a baby is born and takes its first breathes, the ducts close and blood is rerouted to the lungs. DEFINITION The fetal circulation is the circulatory system of a human fetus, often encompassing the entire fetoplacental circulation which includes the umbilical cord and the blood vessels within the placenta that carry fetal blood. PATHWAY Ductus Arteriosus Arch of Aoarta Right Lung Left Atrium Foramen Ovale Right Left AtriumInferior Ventricle Ductus Venosus Venacava Right Ventricle Liver Umbilical Vein Portal Vein Placenta Umbilical Arteries Umbilical Cord 2 umbilical arteries: return non-oxygenated blood, fetal waste, CO2 to placenta 1 umbilical vein: brings oxygenated blood and nutrients to the fetus Foetal circulation consequently differs from the adult one predominantly due to the presence of 3 major vascular shunts: Three shunts are present in fetal life: 1. Ductus venosus: connects the umbilical vein to the inferior vena cava 2. Ductus arteriosus: connects the main pulmonary artery to the aorta 3. Foramen ovale: anatomic opening between the right and left atrium. THE MAIN FUNCTION OF THESE SHUNTS IS TO REDIRECT OXYGENATED BLOOD AWAY FROM THE LUNGS, LIVER AND KIDNEY (WHOSE FUNCTIONS ARE PERFORMED BY THE PLACENTA).