Chapter 3 Fetal Development Key Terms • Viability • Chorion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power
DSJUOG Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology REVIEW ARTICLE Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology 1Ritsuko K Pooh, 2Asim Kurjak 1CRIFM Clinical Research Institute of Fetal Medicine PMC, Osaka, Japan 2Department of Obstetrics and Gynecology, University of Zagreb, School of Medicine, Zagreb, Croatia Correspondence: Ritsuko K Pooh, CRIFM Clinical Research Institute of Fetal Medicine PMC 7-3-7, Uehommachi, Tennoji Osaka #543-0001, Japan, Phone: +81-6-6775-8111, Fax: +81-6-6775-8122, e-mail: [email protected] Abstract Significant advances have been made in accurate and reliable visualization of the cerebral circulation in normal and abnormal pregnancies. They provided the non-invasive studies of fetal cerebral angiogenesis and further development that filled some of the gaps made by neuroanatomical studies alone. The first breakthrough in the assessment of fetal circulation was development of Doppler system with purpose to obtain velocity waveforms. Continuing technical advances in Doppler ultrasound equipment, especially highly sensitive color flow imagining techniques have made it possible to study smaller anatomical parts of fetal circulation system including cerebral vascularization. Before examination of brain vascularity, anatomical vascular structure and development on the different appearance at each gestational age should be remembered as the basic knowledge. Since the development of the embryo is rapid and significant changes occur during even one week it is important to specify the stage of the embryo or fetus both by age (postmenstrual weeks and days) and by size (crownrump length (CRL) and biparietal diameter (BPD). Introduction of three-dimensional (3D) sonography and 3D power Doppler techniques have enabled visualization of intracranial vessels. -

Spawning & Larviculture of Bivalve Mollusks
Spawning & Larviculture of Bivalve Mollusks Grade Level: Subject Area: Time: 9-12 Aquaculture, Biology, Preparation: 30 minutes to prepare Reproduction, Anatomy Activity: 50 minute lecture (may require 1 ½ class periods) Clean-up: NA SPS (SSS): 06.04 Employ correct terminologies for animal species and conditions (e.g. sex, age, etc.) (LA.A.1.4.1-4; LA.A.2.4.4; LA.B.1.4.1-3; LA.B.2.4.1-3; LA.C.1.4.1; LA.C.2.4.1). 11.09 Develop an information file in aquaculture species (LA.A.1.4, 2.4; LA.B.1.4, 2.4; LA.C.1.4, 2.4, 3.4; LA.D.2.4; SC.D.1.4; SC.F.1.4, 2.4; SC.G.1.4, 2.4). 11.10 List and describe the major factors in the growth of aquatic fauna and flora (LA.A.1.4, 2.4; LA.B.1.4, 2.4; LA.C.1.4, 2.4, 3.4; LA.D.2.4; SC.D.1.4, SC.F.1.4, 2.4; SC.G.1.4, 2.4). 13.02 Explain how changes in water affect aquatic life (LA.A.1.4, 2.4; LA.B.2.4; LA.C.1.4, 2.4; LA.D.2.4; SC.F.1.4; SC.G.1.4). 13.03 Explain, monitor, and maintain freshwater/saltwater quality standards for the production of desirable species (LA.A.1.4, 2.4; LA.B.2.4; LA.C.1.4, 2.4; LA.D.2.4; MA>B.1.4; MA.E.1.4, 2.4, 3.4; SC.E.2.4; SC.F.2.4; SC.G.1.4). -

Defective Decidualization During and After Severe Preeclampsia Reveals a Possible Maternal Contribution to the Etiology
Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology Tamara Garrido-Gomeza,b,c,d, Francisco Dominguezb, Alicia Quiñonerob, Patricia Diaz-Gimenob, Mirhan Kapidzicc,d, Matthew Gormleyc,d, Katherine Onac,d, Pablo Padilla-Isertee, Michael McMasterf, Olga Genbacevc,d, Alfredo Peralese,g, Susan J. Fisherc,d,h,i,1,2, and Carlos Simóna,b,g,j,1,2 aFundación Igenomix, 46980 Valencia, Spain; bInstituto Universitario IVI, Instituto de Investigación Sanitaria Hospital Clinico de Valencia INCLIVA, 46010 Valencia, Spain; cCenter for Reproductive Sciences, University of California, San Francisco, CA 94143; dDepartment of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Francisco, CA 94143; eDepartment of Obstetrics and Gynecology, Hospital Universitario La Fe, 46026 Valencia, Spain; fDepartment of Cell and Tissue Biology, University of California, San Francisco, CA 94143; gDepartment of Obstetrics and Gynecology, School of Medicine, Valencia University, 46010 Valencia, Spain; hThe Eli & Edythe Broad Center for Regeneration Medicine and Stem Cell Research, University of California, San Francisco, CA 94143; iDepartment of Anatomy, University of California, San Francisco, CA 94143; and jDepartment of Obstetrics and Gynecology, School of Medicine, Stanford University, Palo Alto, CA 94305 Edited by R. Michael Roberts, University of Missouri-Columbia, Columbia, MO, and approved August 11, 2017 (received for review April 20, 2017) In preeclampsia (PE), cytotrophoblast (CTB) invasion of the uterus in studying the CTB subpopulation that invades the uterine wall in and spiral arteries is often shallow. Thus, the placenta’s role has the context of this syndrome. Targeted analyses of particular mo- been a focus. In this study, we tested the hypothesis that decidual lecular families, such as the vascular-type adhesion molecules that defects are an important determinant of the placental phenotype. -

Chapter 9 Reproduction in Animals.Pmd
9 Reproduction in Animals MULTIPLE CHOICE QUESTIONS 1. Sets of reproductive terms are given below. Choose the set that has an incorrect combination. (a) sperm, testis, sperm duct, penis (b) menstruation, egg, oviduct, uterus (c) sperm, oviduct, egg, uterus (d) ovulation, egg, oviduct, uterus 2. In humans, the development of fertilised egg takes place in the (a) ovary (c) oviduct (b) testis (d) uterus 3. In the list of animals given below, hen is the odd one out. human being, cow, dog, hen The reason for this is (a) it undergoes internal fertilisation. (b) it is oviparous. (c) it is viviparous. (d) it undergoes external fertilisation. 4. Animals exhibiting external fertilisation produce a large number of gametes. Pick the appropriate reason from the following. (a) The animals are small in size and want to produce more offsprings. (b) Food is available in plenty in water. (c) To ensure better chance of fertilisation. (d) Water promotes production of large number of gametes. 5. Reproduction by budding takes place in (a) hydra (c) paramecium (b) amoeba (d) bacteria 6. Which of the following statements about reproduction in humans is correct? (a) Fertilisation takes place externally. 12/04/18 48 EEE XEMPLAR PROBLEMS (b) Fertilisation takes place in the testes. (c) During fertilisation egg moves towards the sperm. (d) Fertilisation takes place in the human female. 7. In human beings, after fertilisation, the structure which gets embedded in the wall of uterus is (a) ovum (c) foetus (b) embryo (d) zygote 8. Aquatic animals in which fertilisation occurs in water are said to be: (a) viviparous without fertilisation. -

Patent Ductus Arteriosus About This Factsheet the Normal Heart
Understanding your child’s heart Patent ductus arteriosus About this factsheet The normal heart This factsheet is for parents of babies and children who The heart is a muscular pump which pumps blood through the have patent ductus arteriosus (PDA), which is also known as body and lungs. There are four chambers in the heart. The two persistent arterial duct. upper ones are called the right atrium and left atrium. These are separated by a wall called the atrial septum. The two lower It explains: chambers are called the right and left ventricles, and are separated • what patent ductus arteriosus is and how it is diagnosed by a wall called the ventricular septum. • how patent ductus arteriosus is treated • the benefits and risks of treatments. On each side of the heart, blood passes from the atrium, through a heart valve – the tricuspid valve on the right, and the mitral valve This factsheet does not replace the advice that doctors or on the left – into the ventricle. The ventricles are the main pumping nurses may give you, but it should help you to understand chambers of the heart. Each ventricle pumps blood out into an artery. what they tell you. The right ventricle pumps blood – blue in the illustration – into the pulmonary artery (the blood vessel that takes blood to the lungs). The left ventricle pumps blood – red in the illustration – into the aorta (the blood vessel that takes blood to the rest of the body). Blood flows from the right side of the heart, through the pulmonary valve into the pulmonary artery, and then to the lungs where it picks up oxygen. -

Anomalies of the Portal Venous System in Dogs and Cats As Seen on Multidetector-Row Computed Tomography: an Overview and Systematization Proposal
veterinary sciences Review Anomalies of the Portal Venous System in Dogs and Cats as Seen on Multidetector-Row Computed Tomography: An Overview and Systematization Proposal Giovanna Bertolini San Marco Veterinary Clinic and Laboratory, via dell’Industria 3, 35030 Veggiano, Padova, Italy; [email protected]; Tel.: +39-049-856-1098 Received: 29 November 2018; Accepted: 16 January 2019; Published: 22 January 2019 Abstract: This article offers an overview of congenital and acquired vascular anomalies involving the portal venous system in dogs and cats, as determined by multidetector-row computed tomography angiography. Congenital absence of the portal vein, portal vein hypoplasia, portal vein thrombosis and portal collaterals are described. Portal collaterals are further discussed as high- and low-flow connections and categorized in hepatic arterioportal malformation, arteriovenous fistula, end-to-side and side-to-side congenital portosystemic shunts, acquired portosystemic shunts, cavoportal and porto-portal collaterals. Knowledge of different portal system anomalies helps understand the underlying physiopathological mechanism and is essential for surgical and interventional approaches. Keywords: portal system; portal vein; portosystemic shunt; portal hypertension; computed tomography 1. Introduction The portal venous system is essential for the maintenance of the liver mass and function in mammals. The portal system collects blood from major abdominal organs (i.e., gastrointestinal tract, pancreas, spleen) delivering nutrients, bacteria and toxins from the intestine to the liver. In addition, the portal blood carries approximately from one-half to two-thirds of the oxygen supply to the liver and specific hepatotrophic factors [1,2]. The portal blood is detoxified by the hepatocytes and then delivered into the systemic circulation via the hepatic veins and caudal vena cava [3]. -

Gonadotrophin Receptors in the Pig Umbilical Cord G
Evidence for the presence of luteinizing hormone\p=n-\chorionic gonadotrophin receptors in the pig umbilical cord G. Wasowicz, K. Derecka, A. Stepien, L. Pelliniemi, T. Doboszynska, B. Gawronska and A. J. Ziecik 'Division ofReproductive Endocrinology, Institute ofAnimal Reproduction and Food Research ofPolish Academy of Sciences, 10-718 Olsztyn, Poland; and 'University of Turku, Kiinamyllynkatu 10, SF 20520 Turku, Finland Pig umbilical cord, like that of humans, contains two arteries and a vein surrounded by Wharton's jelly with amnion covering the exterior surface. The aim of the present study was to investigate whether LH\p=n-\hCGreceptors are present in the pig umbilical cord, using light microscope immunohistochemistry, semiquantitative autoradiography, western blotting and reverse transcription\p=n-\polymerasechain reaction. Umbilical cords were collected on days 48, 71 and 103 of fetal life (n = 6). Monoclonal and polyclonal anti-LH receptor antibodies were used to study receptor distribution. Immunoreactivity was observed in the umbilical blood vessels, the epithelium of umbilical amnion and cells in the Wharton's jelly. No differences in LH\p=n-\hCGreceptor distribution related to the sex of the fetus, period of fetal life or section of the umbilical cord were observed. Strong immunostaining was observed in umbilical vein and in umbilical arteries. However, in the arteries, the tunica media expressed weaker receptor immunostaining than did the tunica intima and tunica adventitia. No immunoactivity was detected in non-target tissue (skeletal muscle) but LH receptors were immunostained in the pig ovary. Topical autoradiography showed that vein and arteries in the umbilical cord bind 125I-labelled hCG, which was highly diminished after co-incubation with an excess of unlabelled hCG. -
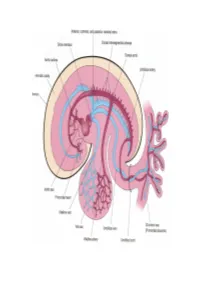
Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

Decidua Produces a Protein That Inhibits Choriogonadotrophin Release from Human Trophoblasts
Decidua produces a protein that inhibits choriogonadotrophin release from human trophoblasts. S G Ren, G D Braunstein J Clin Invest. 1991;87(1):326-330. https://doi.org/10.1172/JCI114990. Research Article To test the hypothesis that uterine decidua may modulate trophoblast function, trophoblasts and decidual cells were isolated from term placentas by enzymatic digestion and Percoll gradient centrifugation. Placental trophoblasts were cocultured with decidual cells and trophoblasts or JEG-3 choriocarcinoma cells were incubated with medium conditioned by decidual cells (DCM) for 72-96 h. In cocultures decidual cells inhibited choriogonadotropin (hCG) release from trophoblasts by 75% in comparison with controls (P less than 0.001). The DCM contained a factor that markedly inhibited hCG release from trophoblasts and JEG cells in vitro compared with controls. The inhibitory effect of the factor on hCG release was dose dependent, and could be eliminated by boiling the DCM for 30 min or proteolytic enzyme treatment. Ultrafiltration and Sephadex G-50 fractionation of the DCM indicated that the apparent molecular mass was 7,000-10,000 D. DCM also inhibited the stimulatory effect of exogenous cAMP on hCG secretion by JEG-3 cells, suggesting that DCM may interfere with activation of the cAMP-dependent protein kinases or transcription of hCG genes. These results suggest that the release of trophoblast hCG is under local paracrine control, regulated in part by a protein released by decidual cells. Find the latest version: https://jci.me/114990/pdf Decidua Produces a Protein that Inhibits Choriogonadotrophin Release from Human Trophoblasts Song-Guang Ren and Glenn D. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -
Development of Right Ventricle
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left.