Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Embryology and Anatomy of Fetal Heart
Prof. Saeed Abuel Makarem Dr. Jamila El Medany Objectives • By the end of this lecture the student should be able to: • Describe the formation, sit, union divisions of the of the heart tubes. • Describe the formation and fate of the sinus venosus. • Describe the partitioning of the common atrium and common ventricle. • Describe the partitioning of the truncus arteriosus. • List the most common cardiac anomalies. • The CVS is the first major system to function in the embryo. • The heart begins to beat at (22nd – 23rd ) days. • Blood flow begins during the beginning of the fourth week and can be visualized by Ultrasound Doppler Notochord: stimulates neural tube formation Somatic mesoderm Splanchnic mesoderm FORMATION OF THE HEART TUBE • The heart is the first functional organ to develop. • It develops from Splanchnic Mesoderm in the wall of the yolk sac (Cardiogenic Area): Cranial to the developing Mouth & Nervous system and Ventral to the developing Pericardial sac. • The heart primordium is first evident at day 18 (as an Angioplastic cords which soon canalize to form the 2 heart tubes). • As the Head Fold completed, the developing heart tubes change their position and become in the Ventral aspect of the embryo, Dorsal to the developing Pericardial sac. • . Development of the Heart tube • After Lateral Folding of the embryo, the 2 heart tubes approach each other and fuse to form a single Endocardial Heart tube within the pericardial sac. • Fusion of the two tubes occurs in a Craniocaudal direction. What is the • The heart tube grows faster than shape of the the pericardial sac, so it shows 5 alternate dilations separated by Heart Tube? constrictions. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

MDCT of Interatrial Septum
Diagnostic and Interventional Imaging (2015) 96, 891—899 PICTORIAL REVIEW /Cardiovascular imaging MDCT of interatrial septum ∗ D. Yasunaga , M. Hamon Service de radiologie, pôle d’imagerie, CHU de Caen, avenue de la Côte-de-Nacre, 14033 Caen Cedex 9, France KEYWORDS Abstract ECG-gated cardiac multidetector row computed tomography (MDCT) allows precise Cardiac CT; analysis of the interatrial septum (IAS). This pictorial review provides a detailed description of Interatrial septum; the normal anatomy, variants and abnormalities of the IAS such as patent foramen ovale, con- Patent foramen genital abnormalities such as atrial septal defects as well as tumors and tumoral-like processes ovale; that develop on the IAS. Secundum ASD © 2015 Published by Elsevier Masson SAS on behalf of the Éditions françaises de radiologie. Introduction Major technical advances in computed tomography (CT) in recent years have made it pos- sible to use multidetector row CT (MDCT) in the field of cardiac imaging. Besides coronary arteries, ECG-gated cardiac MDCT provides high-resolution images of all cardiac structures. It is therefore important for radiologists to understand and be able to analyze the normal anatomical structures, variants and diseases of these different structures. This article provides an analysis of the interatrial septum (IAS) based on a pictorial review. After a short embryological and anatomical description, we will illustrate the nor- mal anatomy and variants of the IAS, anomalies such as patent foramen ovale (PFO), congenital diseases such as atrial septal defects (ASD) as well as tumors and tumoral-like processes that develop on the IAS. Abbreviations: ASA, atrial septal aneurysm; ASD, atrial septal defect; ECG, electrocardiogram; IAS, interatrial septum; IVC, inferior vena cava; IVS, interventricular septum; LV, left ventricle; M, myxoma; PFO, patent foramen ovale; RSPV, right superior pulmonary vein; RV, right ventricle; SVC, superior vena cava; MIP, maximal intensity projection; TEE, transesophageal echocardiography; TV, tricuspid valve. -

Transcriptomic Analysis and Developmental Neural Transcript Identification in the Brittle Star Ophioplocus Esmarki
Rochester Institute of Technology RIT Scholar Works Theses 4-27-2020 Transcriptomic Analysis and Developmental Neural Transcript Identification in the Brittle Star Ophioplocus esmarki Alexandria Shumway [email protected] Follow this and additional works at: https://scholarworks.rit.edu/theses Recommended Citation Shumway, Alexandria, "Transcriptomic Analysis and Developmental Neural Transcript Identification in the Brittle Star Ophioplocus esmarki" (2020). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. 1 Transcriptomic Analysis and Developmental Neural Transcript Identification in the Brittle Star Ophioplocus esmarki By Alexandria Shumway A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Bioinformatics Thomas H. Gosnell School of Life Sciences College of Science Rochester Institute of Technology Rochester, NY April 27, 2020 Rochester Institute of Technology Thomas H. Gosnell School of Life Sciences Bioinformatics Program 2 To: Head, Thomas H. Gosnell School of Life Sciences The undersigned state that Alexandria Juliana Shumway, a candidate for the Master of Science degree in Bioinformatics, has submitted her thesis and has satisfactorily defended it. This completes the requirements for the Master of Science degree in Bioinformatics at Rochester Institute of Technology. Name Date ____________________________ _______________________________ Dr. Hyla Sweet, Ph.D. Thesis Advisor ____________________________ _______________________________ Dr. Michael Osier, Ph.D. Committee Member ____________________________ _______________________________ Dr. Andre Hudson, Ph.D. Committee Member 3 1 ABSTRACT Ophioplocus esmarki is one species within a family of brittle stars that includes an abbreviated mode of development with a non-feeding, vitellaria larva. -

Patent Ductus Arteriosus About This Factsheet the Normal Heart
Understanding your child’s heart Patent ductus arteriosus About this factsheet The normal heart This factsheet is for parents of babies and children who The heart is a muscular pump which pumps blood through the have patent ductus arteriosus (PDA), which is also known as body and lungs. There are four chambers in the heart. The two persistent arterial duct. upper ones are called the right atrium and left atrium. These are separated by a wall called the atrial septum. The two lower It explains: chambers are called the right and left ventricles, and are separated • what patent ductus arteriosus is and how it is diagnosed by a wall called the ventricular septum. • how patent ductus arteriosus is treated • the benefits and risks of treatments. On each side of the heart, blood passes from the atrium, through a heart valve – the tricuspid valve on the right, and the mitral valve This factsheet does not replace the advice that doctors or on the left – into the ventricle. The ventricles are the main pumping nurses may give you, but it should help you to understand chambers of the heart. Each ventricle pumps blood out into an artery. what they tell you. The right ventricle pumps blood – blue in the illustration – into the pulmonary artery (the blood vessel that takes blood to the lungs). The left ventricle pumps blood – red in the illustration – into the aorta (the blood vessel that takes blood to the rest of the body). Blood flows from the right side of the heart, through the pulmonary valve into the pulmonary artery, and then to the lungs where it picks up oxygen. -
Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery
Pediat. Res. 6: 693-700 (1972) Acetylcholine neonate atropine prematurity bradykinin sympathetic nervous system ductus arteriosus The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery INGRID OBERHANSLI-WEISS, MICHAEL A. HEYMANN1391, ABRAHAM M. RUDOLPH, AND KENNETH L. MELMON Cardiovascular Research Institute and Departments of Pediatrics, Physiology and Pharmacology, University of California San Francisco, San Francisco, California, USA Extract Response of the ductus arteriosus and umbilical artery to changes in oxygen tension, to acetylcholine, and to sympathetic and parasympathetic blocking agents was studied in vitro in isolated rings obtained from 22 fetal lambs of 98- to 147-day gestation. After stabilization of tension at a baseline level (0.3-0.7 g) in a PO2 environment of 35-45 mm Hg, both increase of the PO2 to 550 mm Hg and decrease of the PO2 to 8 mm Hg of the bathing solution produced constriction. The mean maximal tension developed by the ductus arteriosus.was 3.91 g at high PO2 and 3.87 g at low POr The increase in maximal tension developed with advancing gestation was also similar at both high and low POj. At P02 levels of 8-550 mm Hg, acetylcholine produced a further increase in tension, whereas bradykinin only produced an increase in tension at high PO2- Alpha and beta sympathetic blockade had no effect on the constrictor response to oxygen. Atropine relaxed the ductus arteriosus and umbilical artery at both high and low Po2 levels; the degree of relaxation was related to drug concentration. Acetylcholin- esterase also relaxed the ductus arteriosus constricted by oxygen. -

6 Development of the Great Vessels and Conduction Tissue
Development of the Great Vessels and Conduc6on Tissue Development of the heart fields • h:p://php.med.unsw.edu.au/embryology/ index.php?6tle=Advanced_-_Heart_Fields ! 2 Septa6on of the Bulbus Cordis Bulbus Cordis AV Canal Ventricle Looking at a sagital sec6on of the heart early in development the bulbus cordis is con6nuous with the ventricle which is con6nuous with the atria. As the AV canal shiOs to the right the bulbus move to the right as well. Septa6on of the Bulbus Cordis A P A P The next three slides make the point via cross sec6ons that the aorta and pulmonary arteries rotate around each other. This means the septum between them changes posi6on from superior to inferior as well. Septa6on of the Bulbus Cordis P A A P Septa6on of the Bulbus Cordis P A P A Migra6on of neural crest cells Neural crest cells migrate from the 3ed, 4th and 6th pharyngeal arches to form some of the popula6on of cells forming the aor6copulmonary septum. Septa6on of the Bulbus Cordis Truncal (Conal) Swellings Bulbus Cordis The cardiac jelly in the region of the truncus and conus adds the neural crest cells and expands as truncal swellings. Septa6on of the Bulbus Cordis Aorticopulmonary septum These swellings grow toward each other to meet and form the septum between the aorta and pulmonary artery. Aorta Pulmonary Artery Septa6on of the Bulbus Cordis Anterior 1 2 3 1 2 3 The aor6copulmonary septum then rotates as it moves inferiorly. However, the exact mechanism for that rota6on remains unclear. Septa6on of the Bulbus Cordis Aorta Pulmonary Artery Conal Ridges IV Foramen Membranous Muscular IV Endocarial Septum Interventricular Cushion Septum However, the aor6copulmonary septum must form properly for the IV septum to be completed. -

Endothelial Dysfunction May Link Interatrial Septal Abnormalities and MTHFR-Inherited Defects to Cryptogenic Stroke Predisposition
biomolecules Article Endothelial Dysfunction May Link Interatrial Septal Abnormalities and MTHFR-Inherited Defects to Cryptogenic Stroke Predisposition 1, 2, 1 1 Luca Sgarra y, Alessandro Santo Bortone y, Maria Assunta Potenza , Carmela Nacci , Maria Antonietta De Salvia 1, Tommaso Acquaviva 2, Emanuela De Cillis 2, Marco Matteo Ciccone 2, Massimo Grimaldi 3 and Monica Montagnani 1,* 1 Department of Biomedical Sciences and Human Oncology—Section of Pharmacology, Medical School, University of Bari “Aldo Moro”, 70124 Bari, Italy; [email protected] (L.S.); [email protected] (M.A.P.); [email protected] (C.N.); [email protected] (M.A.D.S.) 2 Department of Emergency and Organ Transplantation—Section of Cardiovascular Diseases, Medical School, University of Bari “Aldo Moro”, 70124 Bari, Italy; [email protected] (A.S.B.); [email protected] (T.A.); [email protected] (E.D.C.); [email protected] (M.M.C.) 3 General Hospital “F. Miulli” Acquaviva delle Fonti, 70021 Bari, Italy; fi[email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 28 March 2020; Accepted: 2 June 2020; Published: 4 June 2020 Abstract: We explored the significance of the L-Arginine/asymmetric dimethylarginine (L-Arg/ADMA) ratio as a biomarker of endothelial dysfunction in stroke patients. To this aim, we evaluated the correlation, in terms of severity, between the degree of endothelial dysfunction (by L-Arg/ADMA ratio), the methylene tetrahydrofolate reductase (MTHFR) genotype, and the interatrial septum (IAS) phenotype in subject with a history of stroke. Methods and Results: L-Arg, ADMA, and MTHFR genotypes were evaluated; the IAS phenotype was assessed by transesophageal echocardiography. -
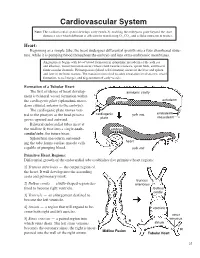
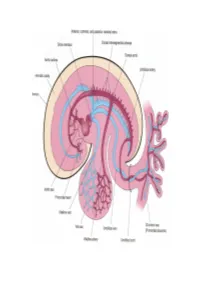
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle.