Embryology and Anatomy of Fetal Heart
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Goals and Outcomes – Gametogenesis, Fertilization (Embryology Chapter 1)
Department of Histology and Embryology, Faculty of Medicine in Pilsen, Charles University, Czech Republic; License Creative Commons - http://creativecommons.org/licenses/by-nc-nd/3.0/ Goals and outcomes – Gametogenesis, fertilization (Embryology chapter 1) Be able to: − Define and use: progenesis, gametogenesis, primordial gonocytes, spermatogonia, primary and secondary spermatocytes, spermatids, sperm cells (spermatozoa), oogonia, primary and secondary oocytes, polar bodies, ovarian follicles (primordial, primary, secondary, tertiary), membrane granulosa, cumulus oophorus, follicular antrum, theca folliculi interna and externa, zona pellucida, corona radiata, ovulation, corpus luteum, corpus albicans, follicular atresia, expanded cumulus, luteinizing hormone (LH), follicle-stimulating hormone (FSH), human chorionic gonadotropin (hCG), sperm capacitation, acrosome reaction, cortical reaction and zona reaction, fertilization, zygote, cleavage, implantation, gastrulation, organogenesis, embryo, fetus, cell division, differentiation, morphogenesis, condensation, migration, delamination, apoptosis, induction, genotype, phenotype, epigenetics, ART – assisted reproductive techniques, spermiogram, IVF-ET (in vitro fertilization followed by embryo transfer), GIFT – gamete intrafallopian transfer, ICSI – intracytoplasmatic sperm injection − Draw and label simplified developmental schemes specified in a separate document. − Give examples of epigenetic mechanisms (at least three of them) and explain how these may affect the formation of phenotype. − Give examples of ethical issues in embryology (at least three of them). − Explain how the sperm cells are formed, starting with primordial gonocytes. Compare the nuclear DNA content, numbers of chromosomes, cell shape and size in all stages. − Explain how the Sertoli cells and Leydig cells contribute to spermatogenesis. − List the parameters used for sperm analysis. What are their normal values? − Explain how the mature oocytes differentiate, starting with oogonia. − Explain how the LH and FSH contribute to oogenesis. -

MDCT of Interatrial Septum
Diagnostic and Interventional Imaging (2015) 96, 891—899 PICTORIAL REVIEW /Cardiovascular imaging MDCT of interatrial septum ∗ D. Yasunaga , M. Hamon Service de radiologie, pôle d’imagerie, CHU de Caen, avenue de la Côte-de-Nacre, 14033 Caen Cedex 9, France KEYWORDS Abstract ECG-gated cardiac multidetector row computed tomography (MDCT) allows precise Cardiac CT; analysis of the interatrial septum (IAS). This pictorial review provides a detailed description of Interatrial septum; the normal anatomy, variants and abnormalities of the IAS such as patent foramen ovale, con- Patent foramen genital abnormalities such as atrial septal defects as well as tumors and tumoral-like processes ovale; that develop on the IAS. Secundum ASD © 2015 Published by Elsevier Masson SAS on behalf of the Éditions françaises de radiologie. Introduction Major technical advances in computed tomography (CT) in recent years have made it pos- sible to use multidetector row CT (MDCT) in the field of cardiac imaging. Besides coronary arteries, ECG-gated cardiac MDCT provides high-resolution images of all cardiac structures. It is therefore important for radiologists to understand and be able to analyze the normal anatomical structures, variants and diseases of these different structures. This article provides an analysis of the interatrial septum (IAS) based on a pictorial review. After a short embryological and anatomical description, we will illustrate the nor- mal anatomy and variants of the IAS, anomalies such as patent foramen ovale (PFO), congenital diseases such as atrial septal defects (ASD) as well as tumors and tumoral-like processes that develop on the IAS. Abbreviations: ASA, atrial septal aneurysm; ASD, atrial septal defect; ECG, electrocardiogram; IAS, interatrial septum; IVC, inferior vena cava; IVS, interventricular septum; LV, left ventricle; M, myxoma; PFO, patent foramen ovale; RSPV, right superior pulmonary vein; RV, right ventricle; SVC, superior vena cava; MIP, maximal intensity projection; TEE, transesophageal echocardiography; TV, tricuspid valve. -

Transcriptomic Analysis and Developmental Neural Transcript Identification in the Brittle Star Ophioplocus Esmarki
Rochester Institute of Technology RIT Scholar Works Theses 4-27-2020 Transcriptomic Analysis and Developmental Neural Transcript Identification in the Brittle Star Ophioplocus esmarki Alexandria Shumway [email protected] Follow this and additional works at: https://scholarworks.rit.edu/theses Recommended Citation Shumway, Alexandria, "Transcriptomic Analysis and Developmental Neural Transcript Identification in the Brittle Star Ophioplocus esmarki" (2020). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. 1 Transcriptomic Analysis and Developmental Neural Transcript Identification in the Brittle Star Ophioplocus esmarki By Alexandria Shumway A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Bioinformatics Thomas H. Gosnell School of Life Sciences College of Science Rochester Institute of Technology Rochester, NY April 27, 2020 Rochester Institute of Technology Thomas H. Gosnell School of Life Sciences Bioinformatics Program 2 To: Head, Thomas H. Gosnell School of Life Sciences The undersigned state that Alexandria Juliana Shumway, a candidate for the Master of Science degree in Bioinformatics, has submitted her thesis and has satisfactorily defended it. This completes the requirements for the Master of Science degree in Bioinformatics at Rochester Institute of Technology. Name Date ____________________________ _______________________________ Dr. Hyla Sweet, Ph.D. Thesis Advisor ____________________________ _______________________________ Dr. Michael Osier, Ph.D. Committee Member ____________________________ _______________________________ Dr. Andre Hudson, Ph.D. Committee Member 3 1 ABSTRACT Ophioplocus esmarki is one species within a family of brittle stars that includes an abbreviated mode of development with a non-feeding, vitellaria larva. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

No Live Individual Homozygous for a Novel Endoglin Mutation Was Found in a Consanguineous Arab Family with Hereditary Haemorrhag
1of4 J Med Genet: first published as 10.1136/jmg.2004.022079 on 1 November 2004. Downloaded from ONLINE MUTATION REPORT No live individual homozygous for a novel endoglin mutation was found in a consanguineous Arab family with hereditary haemorrhagic telangiectasia A Karabegovic*, M Shinawi*, U Cymerman, M Letarte ............................................................................................................................... J Med Genet 2004;41:e119 (http://www.jmedgenet.com/cgi/content/full/41/11/e119). doi: 10.1136/jmg.2004.022079 ereditary haemorrhagic telangiectasia (HHT or Rendu- Osler-Weber syndrome; MIM 187300) is characterised Key points Hby vascular dysplasia and is inherited in an autosomal dominant manner. HHT occurs among many ethnic groups N Mutation analysis was performed in a large Arab over a wide geographical area. Recent epidemiological studies family with a known history of hereditary haemor- have revealed an incidence for this disease of 1 in 5000– rhagic telangiectasia (HHT) and consanguinity. 12 8000. In most cases, the manifestations of HHT are not N A novel exon 7 missense mutation (c.932TRG) in the present at birth, but develop with age; epistaxis is usually the Endoglin (ENG) gene was found in the proband, earliest sign, often occurring in childhood, while mucocuta- suggesting HHT1. neous and gastrointestinal telangiectases develop progres- sively with age.3 Arteriovenous malformations (AVMs) in the N The mutation was present as a single allele in ten pulmonary, cerebral, or hepatic circulations account for some relatives with clinical signs of disease but was absent of the most devastating clinical complications of HHT and are from 21 unaffected family members, indicating that the due to direct connections between arteries and veins.4 The mutation segregates with the phenotype. -

In Situ Detection of Tbx5 Expression in Developing Chick Embryonic Heart
San Jose State University SJSU ScholarWorks Master's Theses Master's Theses and Graduate Research Fall 2010 In Situ Detection Of Tbx5 Expression In Developing Chick Embryonic Heart Vaishali Agarwal San Jose State University Follow this and additional works at: https://scholarworks.sjsu.edu/etd_theses Recommended Citation Agarwal, Vaishali, "In Situ Detection Of Tbx5 Expression In Developing Chick Embryonic Heart" (2010). Master's Theses. 3901. DOI: https://doi.org/10.31979/etd.84mn-c4vy https://scholarworks.sjsu.edu/etd_theses/3901 This Thesis is brought to you for free and open access by the Master's Theses and Graduate Research at SJSU ScholarWorks. It has been accepted for inclusion in Master's Theses by an authorized administrator of SJSU ScholarWorks. For more information, please contact [email protected]. IN SITU DETECTION OF Tbx 5 EXPRESSION IN DEVELOPING EMBRYONIC CHICK HEART A Thesis Presented to The Faculty of the Department of Biological Sciences San Jose State University In Partial Fulfillment of the Requirements for the Degree Master of Science by Vaishali Agarwal December 2010 © 2010 Vaishali Agarwal ALL RIGHTS RESERVED The Designated Thesis Committee Approves the Thesis Titled IN SITU DETECTION OF Tbx 5 EXPRESSION IN DEVELOPING EMBRYONIC CHICK HEART by Vaishali Agarwal APPROVED FOR THE DEPARTMENT OF BIOLOGICAL SCIENCES SAN JOSE STATE UNIVERSITY December 2010 Dr. Steven White Department of Biological Sciences Dr. Michael Sneary Department of Biological Sciences Dr. Bob Fowler Department of Biological Sciences ABSTRACT IN SITU DETECTION OF Tbx 5 EXPRESSION IN DEVELOPING EMBRYONIC CHICK HEART by Vaishali Agarwal The Tbx 5 gene codes for a highly conserved transcription factor containing a DNA-binding motif called the T box (or T-domain). -

Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure
Acta Cardiol Sin 2016;32:731-737 Brief Report doi: 10.6515/ACS20160205A Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure Ali Yildirim,1 Alperen Aydin,2 Tevfik Demir,1 Fatma Aydin,2 Birsen Ucar1 and Zubeyir Kilic1 Background: The aim of the present study was to evaluate the echocardiographic follow-up of patent foramen ovale, which is considered a potential etiological factor in various diseases, and to determine the factors affecting spontaneous closure. Methods: Between January 2000 and June 2012, records of 918 patients with patent foramen ovale were retrospectively reviewed. Patency of less than 3 mm around the fossa ovalis is called patent foramen ovale. Patients with cyanotic congenital heart diseases, severe heart valve disorders and severe hemodynamic left to right shunts were excluded from the study. The patients were divided into three groups based on age; 1 day-1 monthingroup1,1month-12monthsingroup2,andmorethan12monthsingroup3. Results: Of the 918 patients, 564 (61.4%) had spontaneous closure, 328 (35.8%) had patent foramen ovale continued, 15 (1.6%) patients had patent foramen ovale enlarged to 3-5 mm, 6 patients were enlarged to 5-8 mm, and in one patient patent foramen ovale reached to more than 8 mm size. Defect was spontaneously closed in 65.9% of the patients in group 1, 66.7% of the patients in group 2, and 52.3% of the patients in group 3. There was a negative correlation between the age of diagnosis and spontaneous closure (p < 0.05). Gender, prematurity and coexisting malformations such as patent ductus arteriosus and atrial septal aneurysm did not have any effect on spontaneous closure of patent foramen ovale (p > 0.05). -

(Microsoft Powerpoint
6.1. DEVELOPMENT OF MESODERMAL ORGANS Pavel Krejci In vertebrates, the mesoderm becomes partitioned at an early stage into four zones, from medial to lateral: 1. NOTOCHORD : occupies the midline. 2. PARAXIAL MESODERM : future somites. 3. INTERMEDIATE MESODERM : forms gonads, kidneys, and adrenals. 4. LATERAL PLATE MESODERM : the lateral plate is subdivided by the coelom into the outer SOMATIC MESODERM (future limb buds) and inner SPLANCHNIC MESODERM that forms mesenteries and heart. The skeleton originates from three regions: Skull is formed from neural crest ; the vertebrae are formed from somites ; and the bones of the limbs are formed from limb buds and associated lateral plate . SOMITOGENESIS AND MYOGENESIS Somite patterning is a clearest example of segmental arrangement of the vertebrate body. Somites arise in anteroposterior sequence from the paraxial mesoderm by the action of forkhead transcription factors FoxC1 and C2. Somites start as loose cell associations called somitomeres, that later condense into the epithelial somites . This structure is transient as it undergoes epithelial-to- mesenchymal transformation to form the sclerotome (future vertebrae/ribs). Dorsal part of sclerotome forms tendons whereas the lateral part forms dermamyotome that later forms skin (dermatome) and muscles (myotome). Epaxial myotome forms segmental muscles of the body axis, hypaxial myotome forms muscles of the ventral body wall, limbs, and diaphragm. SEGMENTATION MECHANISM: Somites generated by molecular oscillator (a clock) operating in conjunction with a spatial gradient. One cycle of the clock forms one somite, the gradient determines that the somites are formed in anterior to posterior sequence. The clock represents a periodic expression of c-hairy 1, that encodes for transcription factor bHLH in chick. -

Investigating a Microrna-499-5P Network During Cardiac Development
Investigating a microRNA-499-5p network during cardiac development Thesis for a PhD degree Submitted to University of East Anglia by Johannes Gottfried Wittig This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived therefrom must be in accordance with current UK Copyright Law. In addition, any quotation or extract must include full attribution. Principal Investigator: Prof. Andrea Münsterberg Submission Date: 10.05.2019 Declaration of own work Declaration of own work I, Johannes Wittig, confirm that the work for the report with the title: “Investigating a microRNA-499-5p network during cardiac development” was undertaken by myself and that no help was provided from other sources than those allowed. All sections of the report that use quotes or describe an argument or development investigated by other scientist have been referenced, including all secondary literature used, to show that this material has been adopted to support my report. Place/Date Signature II Acknowledgements Acknowledgements I am very happy that I had the chance to be part of the Münsterberg-lab for my PhD research, therefore I would very much like to thank Andrea Münsterberg for offering me this great position in her lab. I especially want to thank her for her patience with me in all the moments where I was impatient and complained about slow progress. I also would like to say thank you for the incredible freedom I had during my PhD work and the support she gave me in the lab but also the understanding for all my non-science related activities. -

Echocardiographic Evaluation of Anatomical Abnormalities Of
Jemds.com Original Research Article Echocardiographic Evaluation of Anatomical Abnormalities of Interventricular Septum (with Special Reference to Ventricular Septal Defect) in the Population of West Bengal between 1 and 12 Years of Age Arpan Kumar Goswami1, Dhruba Mandal2, Shruti Goswami3, Biswajit Majumdar4, Adrija Mandal5 1Assistant Professor, Department of Anatomy, Bankura Sammilani Medical College, Bankura, West Bengal, India. 2Associate Professor, Department of Anatomy, Bankura Sammilani Medical College, Bankura, West Bengal, India. 3Scholar, Tripura Medical College, Agartala, Tripura, India. 4Associate Professor Department of Cardiology, R.G. Kar Medical College, Kolkata, West Bengal, India. 5Scholar, Burdwan Model School, Purba Bardhaman, West Bengal, India. ABSTRACT BACKGROUND Right and left ventricles of human heart are separated by interventricular septum. Corresponding Author: The septum develops in early embryonic period from three sources: primitive Dr. Dhruba Mandal, Rabindrapalli, ventricular septum, bulbar septum and endocardial cushion. Primitive ventricular Burdwan-713101, septum forms the major part of muscular septum, some contribution comes from West Bengal, India. bulbar septum. Endocardial cushion forms the membranous part of ventricular E-mail: [email protected] septum. Obviously, these three parts fuse to form a complete septum. Sometimes they do not fuse properly, or one of them does not proliferate properly, leading to DOI: 10.14260/jemds/2019/777 formation of a defective septum containing one or more foramina. This condition is called ventricular septal defect. Commonly, endocardial cushion does not proliferate Financial or Other Competing Interests: properly producing defect in membranous septum. Less commonly foramina are None. situated in muscular septum. Perimembranous VSD is described as foramen lies in How to Cite This Article: membranous septum and surrounding it, which is due to non-proliferation of Goswami AK, Mandal D, Goswami S, et al. -
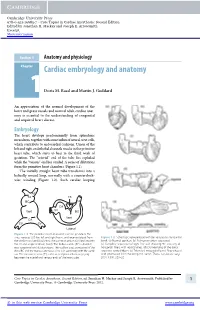
Core Topics in Cardiac Anesthesia, Second Edition, Ed
Cambridge University Press 978-0-521-19685-7 - Core Topics in Cardiac Anesthesia: Second Edition Edited by Jonathan H. Mackay and Joseph E. Arrowsmith Excerpt More information Section 1 Anatomy and physiology Chapter1 Cardiac embryology and anatomy Doris M. Rassl and Martin J. Goddard An appreciation of the normal development of the heart and great vessels and normal adult cardiac anat- omy is essential to the understanding of congenital and acquired heart disease. Embryology The heart develops predominantly from splanchnic mesoderm, together with some influx of neural crest cells, which contribute to endocardial cushions. Union of the left and right endothelial channels results in the primitive heart tube, which starts to beat in the third week of gestation. The “arterial” end of the tube lies cephalad while the “venous” end lies caudad. A series of dilatations form the primitive heart chambers (Figure 1.1). The initially straight heart tube transforms into a helically wound loop, normally with a counterclock- wise winding (Figure 1.2). Such cardiac looping AS TA TS BC TA CA Vent SV BC Vent CA SV SV Lateral Figure 1.1 The primitive heart at around 3 weeks’ gestation. The sinus venosus (SV) has left and right horns, and receives blood from Figure 1.2 Schematic representation of the ventricular myocardial the vitelline and umbilical veins. The common atrium (CA) lies between band. (a) Normal position. (b) Pulmonary artery separated. the SV and single ventricle (Vent). The bulbus cordis (BC) is divided (c) Complete separation of right free wall showing 90 crossing of into a proximal and distal portions.