Anomalies of the Portal Venous System in Dogs and Cats As Seen on Multidetector-Row Computed Tomography: an Overview and Systematization Proposal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anatomy of Th-E Blood Vascular System of the Fox ,Squirrel
THE ANATOMY OF TH-E BLOOD VASCULAR SYSTEM OF THE FOX ,SQUIRREL. §CIURUS NlGER. .RUFIVENTEB (OEOEEROY) Thai: for the 009m of M. S. MICHIGAN STATE COLLEGE Thomas William Jenkins 1950 THulS' ifliillifllfllilllljllljIi\Ill\ljilllHliLlilHlLHl This is to certifg that the thesis entitled The Anatomy of the Blood Vascular System of the Fox Squirrel. Sciurus niger rufiventer (Geoffroy) presented by Thomas William Jenkins has been accepted towards fulfillment of the requirements for A degree in MEL Major professor Date May 23’ 19500 0-169 q/m Np” THE ANATOMY OF THE BLOOD VASCULAR SYSTEM OF THE FOX SQUIRREL, SCIURUS NIGER RUFIVENTER (GEOFFROY) By THOMAS WILLIAM JENKINS w L-Ooffi A THESIS Submitted to the School of Graduate Studies of Michigan State College of Agriculture and Applied Science in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Department of Zoology 1950 \ THESlSfi ACKNOWLEDGMENTS Grateful acknowledgment is made to the following persons of the Zoology Department: Dr. R. A. Fennell, under whose guidence this study was completed; Mr. P. A. Caraway, for his invaluable assistance in photography; Dr. D. W. Hayne and Mr. Poff, for their assistance in trapping; Dr. K. A. Stiles and Dr. R. H. Manville, for their helpful suggestions on various occasions; Mrs. Bernadette Henderson (Miss Mac), for her pleasant words of encouragement and advice; Dr. H. R. Hunt, head of the Zoology Department, for approval of the research problem; and Mr. N. J. Mizeres, for critically reading the manuscript. Special thanks is given to my wife for her assistance with the drawings and constant encouragement throughout the many months of work. -
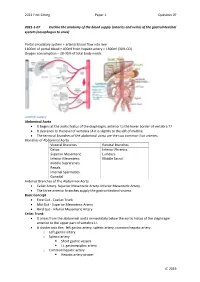
Arteries and Veins) of the Gastrointestinal System (Oesophagus to Anus)
2021 First Sitting Paper 1 Question 07 2021-1-07 Outline the anatomy of the blood supply (arteries and veins) of the gastrointestinal system (oesophagus to anus) Portal circulatory system + arterial blood flow into liver 1100ml of portal blood + 400ml from hepatic artery = 1500ml (30% CO) Oxygen consumption – 20-35% of total body needs Arterial Supply Abdominal Aorta • It begins at the aortic hiatus of the diaphragm, anterior to the lower border of vertebra T7. • It descends to the level of vertebra L4 it is slightly to the left of midline. • The terminal branches of the abdominal aorta are the two common iliac arteries. Branches of Abdominal Aorta Visceral Branches Parietal Branches Celiac. Inferior Phrenics. Superior Mesenteric. Lumbars Inferior Mesenteric. Middle Sacral. Middle Suprarenals. Renals. Internal Spermatics. Gonadal Anterior Branches of The Abdominal Aorta • Celiac Artery. Superior Mesenteric Artery. Inferior Mesenteric Artery. • The three anterior branches supply the gastrointestinal viscera. Basic Concept • Fore Gut - Coeliac Trunk • Mid Gut - Superior Mesenteric Artery • Hind Gut - Inferior Mesenteric Artery Celiac Trunk • It arises from the abdominal aorta immediately below the aortic hiatus of the diaphragm anterior to the upper part of vertebra LI. • It divides into the: left gastric artery, splenic artery, common hepatic artery. o Left gastric artery o Splenic artery ▪ Short gastric vessels ▪ Lt. gastroepiploic artery o Common hepatic artery ▪ Hepatic artery proper JC 2019 2021 First Sitting Paper 1 Question 07 • Left hepatic artery • Right hepatic artery ▪ Gastroduodenal artery • Rt. Gastroepiploic (gastro-omental) artery • Sup pancreatoduodenal artery • Supraduodenal artery Oesophagus • Cervical oesophagus - branches from inferior thyroid artery • Thoracic oesophagus - branches from bronchial arteries and aorta • Abd. -

A Rare Variation of the Inferior Mesenteric Vein with Clinical
CASE REPORT A rare variation of the inferior mesenteric vein with clinical implications Danielle Park, Sarah Blizard, Natalie O’Toole, Sheeva Norooz, Martin Dela Torre, Young Son, Michael McGuinness, Mei Xu Park D, Blizard S, O’Toole N, et al. A rare variation of the inferior the middle colic vein. The superior mesenteric vein then united with the mesenteric vein with clinical implications. Int J Anat Var. Mar 2019;12(1): splenic vein to become the hepatic portal vein. Awareness of this uncommon 024-025. anatomy of the inferior mesenteric vein is important in planning a successful gastrointestinal surgery. Several variations of the inferior mesenteric vein have been previously described. However, this report presents a rare variation that has not yet been noted. In this case, the small inferior mesenteric vein drained into a Key Words: Inferior mesenteric vein; Marginal vein; Middle colic vein; Superior tributary of the marginal vein, which joined the superior mesenteric vein via mesenteric vein INTRODUCTION he portal venous system consists of four large veins: the hepatic portal, Tsplenic (SV), superior mesenteric (SMV) and inferior mesenteric (IMV). The SMV collects the venous return from the small intestine, stomach, pancreas, cecum, ascending colon and proximal portion of the transverse colon. The SMV tributaries include the small intestine, right gastro-omental, inferior pancreaticoduodenal, ileocolic, right colic, middle colic (MCV) and marginal (MarV) veins. The IMV receives the blood from the superior rectal, sigmoid and left colic veins, which cover the distal portion of the transverse colon, descending colon, sigmoid colon and superior rectum. According to the description by Thompson in 1890, the portal vein tributaries are categorized into four types [1]. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Possible Effects of Height of Ligation of the Inferior Mesenteric Vein on Venous Return of the Colorectal Anastomosis: the Venou
Techniques in Coloproctology (2019) 23:799–800 https://doi.org/10.1007/s10151-019-02038-2 VIDEO FORUM Possible efects of height of ligation of the inferior mesenteric vein on venous return of the colorectal anastomosis: the venous trunk theory A. García‑Granero1,2 · G. Pellino1,3 · M. Frasson1 · V. Primo Romaguera1 · D. Fletcher‑Sanfeliu4 · A. Blasco Serra2 · A. A. Valverde‑Navarro2 · F. Martinez‑Soriano2 · E. García‑Granero1 Received: 10 June 2019 / Accepted: 8 July 2019 / Published online: 18 July 2019 © Springer Nature Switzerland AG 2019 Poor arterial vascularization is an independent predictor A detailed demonstration of the arterial and venous vas- of anastomotic failure after rectal resection with colorec- cularization of the left colon is shown. The venous return tal anastomosis [1]. However, there are little data available drains in two ways: via the middle colic vein (through the about the role of venous ischemia in anastomotic failure and marginal arch), and the IMV. The main tributary veins of the how the risk of venous ischemia can be reduced. Ligation of IMV are the sigmoid vein and the left colic vein. Usually, the inferior mesenteric vein (IMV) makes it possible to gain the IMV and the sigmoid vein join in a single venous trunk length and to reduce the tension of the colorectal anastomo- before draining into the left colic vein [4]. An anterior resec- sis [2]. Nevertheless, some authors state that this might be tion of the rectum with high tie of the inferior mesenteric responsible for increased venous stasis, thereby increasing artery (IMA) is simulated. The left colic artery and IMV the risk of venous ischemia of the colorectal anastomosis are ligated near to the IMA stump. -

Prevalence and Outcome of Absence of Ductus Venosus at 11+0 to 13+6 Weeks
Original Paper Fetal Diagn Ther 2011;30:35–40 Received: November 17, 2010 DOI: 10.1159/000323593 Accepted after revision: December 14, 2010 Published online: February 19, 2011 Prevalence and Outcome of Absence of Ductus Venosus at 11+0 to 13 +6 Weeks a, c a a a Ismini Staboulidou Susana Pereira Jader de Jesus Cruz Argyro Syngelaki a, b Kypros H. Nicolaides a b Harris Birthright Research Centre of Fetal Medicine, King’s College Hospital, and Fetal Medicine Unit, University c College Hospital, London , UK; Department of Gynecology and Obstetrics, University Medical School of Hannover, Hannover , Germany Key Words Introduction ؒ Agenesis of ductus venosus ؒ Nuchal translucency First-trimester screening ؒ Prenatal diagnosis The ductus venosus (DV) plays an important role in the fetal circulation because it diverts oxygenated blood from the placenta towards the right atrium and through Abstract the foramen ovale to the left heart and thereafter the Introduction: To examine the prevalence and outcome of ab- brain [1] . Previous studies have examined pregnancy out- sent ductus venosus (DV) diagnosed at 11–13 weeks’ gesta- come in fetuses with absent DV diagnosed during the tion. Method: Prospective screening study for aneuploidies second and third trimester of pregnancy ( table 1 ) [2–27] . in 65,840 singleton pregnancies, including measurement of In the combined data from 26 reports on a total of 110 nuchal translucency (NT) thickness and examination of the cases, about 40% had associated defects and aneuploidies. DV. Prenatal findings and outcome of fetuses with absent DV In the pregnancies with isolated absent DV, about 35% were examined. -

Inferior Mesenteric Artery Abdominal Aorta
Gastro-intestinal Module Dr. Gamal Taha Abdelhady Assistant Professor of Anatomy & Embryology Blood Supply of the GIT Basic Concept ◼ Fore Gut ◼ Celiac Trunk ◼ Mid Gut ◼ Superior Mesenteric Artery ◼ Hind Gut ◼ Inferior Mesenteric Artery Abdominal Aorta ◼ It begins at the aortic hiatus of the diaphragm, anterior to the lower border of vertebra T12. ◼ It descends to the level of vertebra L4 it is slightly to the left of midline. ◼ The terminal branches of the abdominal aorta are the two common iliac arteries. Branches of Abdominal Aorta ◼ Visceral Branches ◼ Parietal Branches 1. Celiac (1). 2. Superior Mesenteric 1. Inferior Phrenics (1). (2). 3. Inferior Mesenteric 2. Lumbar arteries (1). 4. Middle Suprarenals 3. Middle Sacral (1). (2). 5. Renal arteries (2). 6. Gonadal arteries (2) Anterior Branches of The Abdominal Aorta 1. Celiac Artery. 2. Superior Mesenteric Artery. 3. Inferior Mesenteric Artery. ◼ The three anterior branches supply the gastrointestinal viscera. Celiac Trunk ◼ It arises from the abdominal aorta immediately below the aortic hiatus of the diaphragm anterior to the upper part of vertebra L1. ◼ It divides into the: ◼ Left gastric artery, ◼ Splenic artery, ◼ Common hepatic artery. Celiac Trunk • LEFT GASTRIC ARTERY: Lower part of esophagus and lesser curve of stomach • SPLENIC ARTERY – Short gastric vessels – Lt. gastroepiploic artery • COMMON HEPATIC ARTERY – Hepatic artery proper • Left hepatic artery • Right hepatic artery – Gastroduodenal artery gives off Rt. Gastroepiploic (gastro-omental ) artery and Superior pancreatoduodenal artery “Supra-duodenal artery” Superior Mesenteric Artery • It arises from the abdominal aorta immediately 1cm below the celiac artery anterior to the lower part of vertebra L1. • It is crossed anterior by the splenic vein and the neck of pancreas. -
Nervous and Vascular System
NO. A100 KEY CHART FOR MODEL NERVOUS AND VASCULAR SYSTEM 神経系・循環系・門脈系 模型 MADE IN JAPAN KEY CHART FOR MODEL NO. A100 NERVOUS AND VASCULAR SYSTEM 神経系・循環系・門脈系模型 White labels BRAIN ENCEPHALON 脳 A.Frontal lobe of cerebrum A. Lobus frontalis A. 前頭葉 1. Marginal gyrus 1. Gyrus frontalis superior 1. 上前頭回 2. Middle frontal gyrus 2. Gyrus frontalis medius 2. 中前頭回 3. Inferior frontal gyrus 3. Gyrus frontalis inferior 3. 下前頭回 4. Precentral gyru 4. Gyrus precentralis 4. 中心前回 B. Parietal lobe of cerebrum B. Lobus parietalis B. 全頂葉 5. Postcentral gyrus 5. Gyrus postcentralis 5. 中心後回 6. Superior parietal lobule 6. Lobulus parietalis superior 6. 上頭頂小葉 7. Inferior parietal lobule 7. Lobulus parietalis inferior 7. 下頭頂小葉 C.Occipital lobe of cerebrum C. Lobus occipitalis C. 後頭葉 D. Temporal lobe D. Lobus temporalis D. 側頭葉 8. Superior temporal gyrus 8. Gyrus temporalis superior 8. 上側頭回 9. Middle temporal gyrus 9. Gyrus temporalis medius 9. 中側頭回 10. Inferior temporal gyrus 10. Gyrus temporalis inferior 10. 下側頭回 11. Lateral sulcus 11. Sulcus lateralis 11. 外側溝(外側大脳裂) E. Cerebellum E. Cerebellum E. 小脳 12. Biventer lobule 12. Lobulus biventer 12. 二腹小葉 13. Superior semilunar lobule 13. Lobulus semilunaris superior 13. 上半月小葉 14. Inferior lobulus semilunaris 14. Lobulus semilunaris inferior 14. 下半月小葉 15. Tonsil of cerebellum 15. Tonsilla cerebelli 15. 小脳扁桃 16. Floccule 16. Flocculus 16. 片葉 F.Pons F. Pons F. 橋 G.Medullary G. Medulla oblongata G. 延髄 SPINAL CORD MEDULLA SPINALIS 脊髄 H. Cervical enlargement H.Intumescentia cervicalis H. 頸膨大 I.Lumbosacral enlargement I. Intumescentia lumbalis I. 腰膨大 J.Cauda equina J. -

6 Development of the Great Vessels and Conduction Tissue
Development of the Great Vessels and Conduc6on Tissue Development of the heart fields • h:p://php.med.unsw.edu.au/embryology/ index.php?6tle=Advanced_-_Heart_Fields ! 2 Septa6on of the Bulbus Cordis Bulbus Cordis AV Canal Ventricle Looking at a sagital sec6on of the heart early in development the bulbus cordis is con6nuous with the ventricle which is con6nuous with the atria. As the AV canal shiOs to the right the bulbus move to the right as well. Septa6on of the Bulbus Cordis A P A P The next three slides make the point via cross sec6ons that the aorta and pulmonary arteries rotate around each other. This means the septum between them changes posi6on from superior to inferior as well. Septa6on of the Bulbus Cordis P A A P Septa6on of the Bulbus Cordis P A P A Migra6on of neural crest cells Neural crest cells migrate from the 3ed, 4th and 6th pharyngeal arches to form some of the popula6on of cells forming the aor6copulmonary septum. Septa6on of the Bulbus Cordis Truncal (Conal) Swellings Bulbus Cordis The cardiac jelly in the region of the truncus and conus adds the neural crest cells and expands as truncal swellings. Septa6on of the Bulbus Cordis Aorticopulmonary septum These swellings grow toward each other to meet and form the septum between the aorta and pulmonary artery. Aorta Pulmonary Artery Septa6on of the Bulbus Cordis Anterior 1 2 3 1 2 3 The aor6copulmonary septum then rotates as it moves inferiorly. However, the exact mechanism for that rota6on remains unclear. Septa6on of the Bulbus Cordis Aorta Pulmonary Artery Conal Ridges IV Foramen Membranous Muscular IV Endocarial Septum Interventricular Cushion Septum However, the aor6copulmonary septum must form properly for the IV septum to be completed. -

Ductus Venosus
Chapter 28 Ductus Venosus Torvid Kiserud The ductus venosus (venous duct, ductus Arantii) is pography. The thoracic IVC is short or non-existent one of the three physiological shunts responsible for in the human fetus and there is no valve developed at the circulatory adaptation to intrauterine life. It is at- the ductus venosus outlet, the effect being that the tributed to Giulio Cesare Aranzi (1530±1589), but the ductus venosus projects the blood flow directly to- first written account dates back to his contemporary wards the foramen ovale from a short distance and is Vesalius in 1561 [1]. Its function was long recognized less dependent on laminar flow arrangement to avoid [2, 3] but of hardly any clinical importance until ul- extensive blending with low oxygenated blood from trasound techniques were introduced [4±6]. It is now the abdominal IVC [13]. widely used as an important part of the hemody- During early gestation, the ductus venosus is namic assessment of the fetus [7] and has been sug- formed as a confluence of hepatic sinuses, then devel- gested for diagnostic use after birth as well [8]. Anatomy and Development The ductus venosus is a thin, slightly trumpet-shaped vessel connecting the intra-abdominal umbilical vein with the inferior vena cava (IVC; Fig. 28.1). Its inlet, the isthmus, is on average 0.7 mm at 18 weeks and 1.7 mm at 40 weeks of gestation [9±11]. It leaves the umbilical vein (portal sinus) in a cranial and dorsal direction and reaches the IVC at the level of the he- patic venous confluence shortly below the atria. -

Portal Hypertension: the Desperate Search for the Placenta
Current Research in Translational Medicine 67 (2019) 56–61 Available online at ScienceDirect www.sciencedirect.com Original article Portal hypertension: The desperate search for the placenta a, b c,d c,d Maria Angeles Aller *, Natalia Arias , Javier Blanco-Rivero , Gloria Balfagón , a Jaime Arias a Department of Surgery. School of Medicine, Complutense University of Madrid, 28040 Madrid, Spain b Upper Third Floor, UCL Medical School, UCL Institute for Liver and Digestive Health, Royal Free Hospital, Rowland Hill Street, London NWÁ 2PF, UK c Department of Physiology, School of Medicine, Autónoma University of Madrid, Spain d Instituto de Investigación Sanitaria del Hospital Universitario La Paz (IdiPAZ), Madrid, Spain A R T I C L E I N F O A B S T R A C T Article history: We propose that the circulatory impairments produced, in both portal hypertension and liver cirrhosis, to Received 26 February 2018 a certain degree resemble those characterizing prenatal life in the fetus. In fact, the left-right circulatory Accepted 30 September 2018 syndrome is common in cirrhotic patients and in the fetus. Thus, in patients with portal hypertension and Available online 30 November 2018 chronic liver failure, the re-expression of a blood circulation comparable to fetal circulation is associated with the development of similar amniotic functions, i.e., ascites production and placenta functions, and Keywords: portal vascular enteropathy. Therefore, these re-expressed embryonic functions are extra-embryonic and Portal hypertension responsible for prenatal trophism and development. Portosystemic shunts Placenta © 2018 Published by Elsevier Masson SAS. Hyperdynamic circulation Left-right shunts 1. Introduction esophagus and upper stomach, the splenorenal veins, the para- rectal veins with haemorrhoids and the paraumbilical veins, which Increased venous pressure in the splanchnic venous system can result in dilated veins in the anterior abdominal wall, thus making reach pathological levels and induce complications, which in turn up a “caput medusa” [1,2]. -

[email protected]
Embryology)**@gmail.com 1-To serve prenatal needs. 2-To permit modifications at birth, which establish the neonatal circulation #Remember : Good respiration in the newborn infant is dependent upon normal circulatory changes at birth. Three structures are very important in the transitional circulation: 1- Ductus venosus. 2- Ductus arteriosus. 3- Foramen ovale Embryology)**@gmail.com Blood reaches & leaves the fetus through the umbilical cord and it Contains two arteries and one vein. 1- Highly oxygenated blood passes from the placenta through the umbilical vein. 2-Half of this blood reaches the IVC through the ductus venosus. 3- The other half passes to liver sinusoids then to the IVC. 4- Blood of the IVC reaches the right atrium, then left atrium through the Foramen Ovale (an opening between the two atrium). 5- Then to the left ventricle to the ascending aorta, and the aortic arch to supply head & neck brain, cardiac muscle and upper limbs with highly oxygenated blood. 6- Small amount of highly oxygenated blood in right atrium mixes with venous blood of the SVC passes to right ventricle. 7- Then to the pulmonary artery (lungs are not functioning yet) then to ductus arteriosus to the descending aorta, to lower half of fetal body. 8- Then back to placenta via the two umbilical arteries. Embryology)**@gmail.com 12 # After Ligation of the umbilical cord there will be Sudden fall of blood pressure in the IVC and the right Atrium Also the valve of the ductus venosus constricts. # After Aeration ( ventilation ) of the lungs at birth: 1- Marked increase in the pulmonary blood flow due to functioning of the lungs and increase pressure in left atrium causing physiological colsure.