Lymphspiration Are There Lymphatic Vessels in the Placenta?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power
DSJUOG Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology REVIEW ARTICLE Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology 1Ritsuko K Pooh, 2Asim Kurjak 1CRIFM Clinical Research Institute of Fetal Medicine PMC, Osaka, Japan 2Department of Obstetrics and Gynecology, University of Zagreb, School of Medicine, Zagreb, Croatia Correspondence: Ritsuko K Pooh, CRIFM Clinical Research Institute of Fetal Medicine PMC 7-3-7, Uehommachi, Tennoji Osaka #543-0001, Japan, Phone: +81-6-6775-8111, Fax: +81-6-6775-8122, e-mail: [email protected] Abstract Significant advances have been made in accurate and reliable visualization of the cerebral circulation in normal and abnormal pregnancies. They provided the non-invasive studies of fetal cerebral angiogenesis and further development that filled some of the gaps made by neuroanatomical studies alone. The first breakthrough in the assessment of fetal circulation was development of Doppler system with purpose to obtain velocity waveforms. Continuing technical advances in Doppler ultrasound equipment, especially highly sensitive color flow imagining techniques have made it possible to study smaller anatomical parts of fetal circulation system including cerebral vascularization. Before examination of brain vascularity, anatomical vascular structure and development on the different appearance at each gestational age should be remembered as the basic knowledge. Since the development of the embryo is rapid and significant changes occur during even one week it is important to specify the stage of the embryo or fetus both by age (postmenstrual weeks and days) and by size (crownrump length (CRL) and biparietal diameter (BPD). Introduction of three-dimensional (3D) sonography and 3D power Doppler techniques have enabled visualization of intracranial vessels. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

Lymphangiogenesis and Angiogenesis During Human Fetal
Roost et al. Vascular Cell 2014, 6:22 http://www.vascularcell.com/content/6/1/22 VASCULAR CELL RESEARCH Open Access Lymphangiogenesis and angiogenesis during human fetal pancreas development Matthias S Roost1, Liesbeth van Iperen1, Ana de Melo Bernardo1, Christine L Mummery1, Françoise Carlotti2, Eelco JP de Koning2,3 and Susana M Chuva de Sousa Lopes1,4* Abstract Background: The complex endocrine and exocrine functionality of the human pancreas depends on an efficient fluid transport through the blood and the lymphatic vascular systems. The lymphatic vasculature has key roles in the physiology of the pancreas and in regulating the immune response, both important for developing successful transplantation and cell-replacement therapies to treat diabetes. However, little is known about how the lymphatic and blood systems develop in humans. Here, we investigated the establishment of these two vascular systems in human pancreas organogenesis in order to understand neovascularization in the context of emerging regenerative therapies. Methods: We examined angiogenesis and lymphangiogenesis during human pancreas development between 9 and 22 weeks of gestation (W9-W22) by immunohistochemistry. Results: As early as W9, the peri-pancreatic mesenchyme was populated by CD31-expressing blood vessels as well as LYVE1- and PDPN-expressing lymphatic vessels. The appearance of smooth muscle cell-coated blood vessels in the intra-pancreatic mesenchyme occurred only several weeks later and from W14.5 onwards the islets of Langerhans also became heavily irrigated by blood vessels. In contrast to blood vessels, LYVE1- and PDPN-expressing lymphatic vessels were restricted to the peri-pancreatic mesenchyme until later in development (W14.5-W17), and some of these invading lymphatic vessels contained smooth muscle cells at W17. -

The Rediscovery of the Lymphatic System: Old and New Insights Into the Development and Biological Function of the Lymphatic Vasculature
Downloaded from genesdev.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature Guillermo Oliver1,3 and Michael Detmar2,3 1Department of Genetics, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105, USA; 2Cutaneous Biology Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114, USA The lymphatic system is composed of a vascular net- control lymphatic development and function. These work of thin-walled capillaries that drain protein-rich findings include the identification of specific genetic de- lymph from the extracellular spaces within most organs. fects in certain hereditary diseases that are associated A continuous single-cell layer of overlapping endothelial with lymphatic hypoplasia and dysfunction (i.e., lymph- cells lines the lymphatic capillaries, which lack a con- edemas; Milroy 1892; Meige 1898), and evidence that tinuous basement membrane and are, therefore, highly malignant tumors can directly activate lymphangiogen- permeable. Lymph returns to venous circulation via the esis and lymphatic metastasis (Karpanen et al. 2001; larger lymphatic collecting vessels, which contain a Mandriota et al. 2001; Skobe et al. 2001a; Stacker et al. muscular and adventitial layer, and the thoracic duct. 2001). The lymphatic system also includes lymphoid organs such as the lymph nodes, tonsils, Peyer’s patches, spleen, -
Development of Right Ventricle
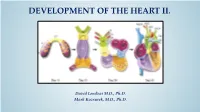
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

The Levels of Angiogenic and Anti-Angiogenic Molecule Concentrations in Pregnancy Based Disorders in the Maternal and Fetal Circ
Master of Philosophy THE LEVELS OF ANGIOGENIC AND ANTI-ANGIOGENIC MOLECULE CONCENTRATIONS IN PREGNANCY BASED DISORDERS IN THE MATERNAL AND FETAL CIRCULATION Islam Afzal Supervisor: Professor Asif Ahmed Department of Reproductive and Vascular Biology November 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Islam Afzal Contents Table of Figures ............................................................................................... 5 Abbreviations ................................................................................................... 7 Acknowledgements .......................................................................................... 9 Abstract .......................................................................................................... 10 Introduction .................................................................................................... 11 Preeclampsia ................................................................................................ -

Has a Role in Cardiovascular and Placental Development and Is a Binding Partner of the Α4 Integrin
Abl-interactor-1 (Abi1) has a role in cardiovascular and placental development and is a binding partner of the α4 integrin Colleen Ringa,1, Mark H. Ginsbergb, Jacob Halingb, and Ann Marie Pendergasta,1 aDepartment of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, NC 27710; and bDepartment of Medicine, University of California at San Diego, La Jolla, CA 92093 Edited* by Stephen P. Goff, Columbia University College of Physicians and Surgeons, New York, NY, and approved November 30, 2010 (received for review August 19, 2010) Dynamic signals linking the actin cytoskeleton and cell adhesion properties among integrin subunits in that α4 predominantly receptors are essential for morphogenesis during development accumulates at the leading edge of migrating cells, rather than at and normal tissue homeostasis. Abi1 is a central regulator of actin focal adhesions. Moreover, α4 expression is associated with polymerization through interactions with multiple protein com- protrusive activity and enhanced cell migration; however, the plexes. However, the in vivo role of Abi1 remains to be defined. pathways that link α4 integrin to the actin-regulatory machinery The α4 integrin adhesion receptor is associated with enhanced at the leading edge have remained elusive. Here we identify Abi1 protrusive activity and regulation of directional cell migration. as a target of α4 integrin that positively regulates membrane Among integrin subunits, α4 exhibits unique properties in that it protrusion by promoting actin polymerization at sites of integrin predominantly accumulates at the leading edge of migrating cells; engagement. however, the pathways that link the actin-regulatory machinery to The Abi family proteins, Abi1 and Abi2, were originally id- α4 at the leading edge have remained elusive. -

Lymphatic Tissue Engineering and Regeneration Laura Alderfer1, Alicia Wei1 and Donny Hanjaya-Putra1,2,3,4,5,6*
Alderfer et al. Journal of Biological Engineering (2018) 12:32 https://doi.org/10.1186/s13036-018-0122-7 REVIEW Open Access Lymphatic Tissue Engineering and Regeneration Laura Alderfer1, Alicia Wei1 and Donny Hanjaya-Putra1,2,3,4,5,6* Abstract The lymphatic system is a major circulatory system within the body, responsible for the transport of interstitial fluid, waste products, immune cells, and proteins. Compared to other physiological systems, the molecular mechanisms and underlying disease pathology largely remain to be understood which has hindered advancements in therapeutic options for lymphatic disorders. Dysfunction of the lymphatic system is associated with a wide range of disease phenotypes and has also been speculated as a route to rescue healthy phenotypes in areas including cardiovascular disease, metabolic syndrome, and neurological conditions. This review will discuss lymphatic system functions and structure, cell sources for regenerating lymphatic vessels, current approaches for engineering lymphatic vessels, and specific therapeutic areas that would benefit from advances in lymphatic tissue engineering and regeneration. Keywords: Lymphangiogenesis, Tissue Engineering, Disease Modeling, Wound Healing, Lymphedema, Stem Cells, Biomaterials, Interstitial Fluid, Regeneration I. Introduction to the Lymphatic System and its role Interstitial fluid (IF) is a plasma filtrate that is generated Function by transcapillary filtration and is governed by Starling The lymphatic system is nearly ubiquitous in the human forces, the net difference between hydrostatic and body, present in all tissues except the epidermis, cartil- osmotic pressures, at the microcirculatory level [9]. In age, eye lens, cornea, retina, and bone marrow [1, 2]. order to maintain fluid homeostasis, lymph formation in The main functions of the lymphatic system include the initial lymphatic vessels must be balanced by the net fluid homeostasis and interstitial fluid drainage, immune flux of plasma being filtered out [4]. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Equine Placenta – Marvelous Organ and a Lethal Weapon
Equine placenta – marvelous organ and a lethal weapon Malgorzata Pozor, DVM, PhD, Diplomate ACT Introduction Placenta has been defined as: „an apposition between parent (usually maternal) and fetal tissue in order to establish physiological exchange” (1). Another definition of this important organ was proposed by Steven and Morris: „a device consisting of one or more transport epithelia located between fetal and maternal blood supply” (2). The main function of placenta is to provide an interface between the dam and the the fetus and to allow the metabolic exchange of the the nutrients, oxygen and waste material. The maternal circulation is brought into a close apposition to the fetal circulation, while a separation of these two circulatory systems remain separated (3). A degree and complexity of this „intimate relationship” varies greately between species mostly due to the structural diversity of the extraembryonic membranes of the vertebrates. The early feto-maternal exchange in the equine pregnancy is established as early as on day 22 after fertilization. The fetal and choriovitellin circulations are already present, the capsule ruptures and the allantois is already visible (4). The allantois starts expanding by day 32 and vascularizes approximately 90% of the chorion and fuses with it to form chorioallantois by day 38 of gestation (5). The equine placenta continues increasing its complexity till approximately day 150 of gestation. Equids have epitheliochorial placenta, there are six leyers separating maternal and fetal circulation, and there are no erosion of the luminal, maternal epithelium, like in ruminants (6). Thousands of small chorionic microvilli develop and penetrate into endometrial invaginations.