Equine Placenta – Marvelous Organ and a Lethal Weapon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

CCM2 and CCM3 Proteins Contribute to Vasculogenesis and Angiogenesis in Human Placenta
Histol Histopathol (2009) 24: 1287-1294 Histology and http://www.hh.um.es Histopathology Cellular and Molecular Biology CCM2 and CCM3 proteins contribute to vasculogenesis and angiogenesis in human placenta Gamze Tanriover1, Yasemin Seval1, Leyla Sati1, Murat Gunel2 and Necdet Demir1 1Department of Histology and Embryology, Akdeniz University, School of Medicine, Antalya, Turkey and 2 Department of Neurosurgery, Yale University, School of Medicine, New Haven, CT, USA Summary. Placenta as an ideal model to study Introduction angiogenic mechanisms have been established in previous studies. There are two processes, The placenta is a multifaceted organ that plays a vasculogenesis and angiogenesis, involved in blood critical role in maintaining and protecting the developing vessel formation during placental development. fetus. Normal development and function of the placenta Therefore, blood vessel formation is a crucial issue that requires extensive vasculogenesis and subsequent might cause vascular malformations. One of the vascular angiogenesis, in both maternal and fetal tissues. malformations is cerebral cavernous malformation Vasculogenesis is the formation of the primitive vascular (CCM) in the central nervous system, consisting of network de novo from progenitor cells, and angiogenesis endothelium-lined vascular channels without intervening is identified as the extension of blood vessels from normal brain parenchyma. Three CCM loci have been preexisting vascular structures (Demir et al., 1989, 2006; mapped as Ccm1, Ccm2, Ccm3 genes in CCM. In order Geva et al., 2002; Charnock-Jones et al., 2004). Many to investigate whether CCM proteins participate in blood factors, such as vascular endothelial growth factor vessel formation, we report here the expression patterns (VEGF), angiopoietins (Angpt-1 and -2) and their of CCM2 and CCM3 in developing and term human receptors are involved in the molecular regulation of placenta by means of immunohistochemistry and these diverse developmental steps. -

Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power
DSJUOG Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology REVIEW ARTICLE Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology 1Ritsuko K Pooh, 2Asim Kurjak 1CRIFM Clinical Research Institute of Fetal Medicine PMC, Osaka, Japan 2Department of Obstetrics and Gynecology, University of Zagreb, School of Medicine, Zagreb, Croatia Correspondence: Ritsuko K Pooh, CRIFM Clinical Research Institute of Fetal Medicine PMC 7-3-7, Uehommachi, Tennoji Osaka #543-0001, Japan, Phone: +81-6-6775-8111, Fax: +81-6-6775-8122, e-mail: [email protected] Abstract Significant advances have been made in accurate and reliable visualization of the cerebral circulation in normal and abnormal pregnancies. They provided the non-invasive studies of fetal cerebral angiogenesis and further development that filled some of the gaps made by neuroanatomical studies alone. The first breakthrough in the assessment of fetal circulation was development of Doppler system with purpose to obtain velocity waveforms. Continuing technical advances in Doppler ultrasound equipment, especially highly sensitive color flow imagining techniques have made it possible to study smaller anatomical parts of fetal circulation system including cerebral vascularization. Before examination of brain vascularity, anatomical vascular structure and development on the different appearance at each gestational age should be remembered as the basic knowledge. Since the development of the embryo is rapid and significant changes occur during even one week it is important to specify the stage of the embryo or fetus both by age (postmenstrual weeks and days) and by size (crownrump length (CRL) and biparietal diameter (BPD). Introduction of three-dimensional (3D) sonography and 3D power Doppler techniques have enabled visualization of intracranial vessels. -

Mechanism of Wound-Healing Activity of Hippophae Rhamnoides L. Leaf Extract in Experimental Burns
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2011, Article ID 659705, 9 pages doi:10.1093/ecam/nep189 Original Article Mechanism of Wound-Healing Activity of Hippophae rhamnoides L. Leaf Extract in Experimental Burns Nitin K. Upadhyay,1 Ratan Kumar,1 M. S. Siddiqui,2 and Asheesh Gupta1 1 Defence Institute of Physiology and Allied Science, DRDO, Delhi 110054, India 2 Department of Toxicology, Jamia Hamdard, Delhi 110062, India Correspondence should be addressed to Asheesh Gupta, [email protected] Received 20 July 2009; Accepted 19 October 2009 Copyright © 2011 Nitin K. Upadhyay et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The present investigation was undertaken to evaluate the healing efficacy of lyophilized aqueous leaf extract of Sea buckthorn (Hippophae rhamnoides L., family Elaeagnaceae) (SBT) and to explore its possible mechanism of action on experimental burn wounds in rats. The SBT extract, at various concentrations, was applied topically, twice daily for 7 days. Treatment with silver sulfadiazine (SSD) ointment was used as reference control. The most effective concentration of the extract was found to be 5.0% (w/w) for burn wound healing and this was further used for detailed study. The SBT-treated group showed faster reduction in wound area in comparison with control and SSD-treated groups. The topical application of SBT increased collagen synthesis and stabilization at the wound site, as evidenced by increase in hydroxyproline, hexosamine levels and up-regulated expression of collagen type-III. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Messages from the Placentae Across Multiple Species a 50 Years
Placenta 84 (2019) 14–27 Contents lists available at ScienceDirect Placenta journal homepage: www.elsevier.com/locate/placenta Messages from the placentae across multiple species: A 50 years exploration T Hiroaki Soma Saitama Medical University, Japan ARTICLE INFO ABSTRACT Keywords: This review explores eight aspects of placentation in multiple mammalian. Gestational trophoblastic disease 1) Specialities of gestational trophoblastic disease. SUA(Single umbilical artery) 2) Clinical significance of single umbilical artery (SUA) syndrome. DIC(Disseminated intravascular coagulation) in 3) Pulmonary trophoblast embolism in pregnant chinchillas and DIC in pregnant giant panda. giant panda 4) Genetics status and placental behaviors during Japanese serow and related antelopes. Placentation in Japanese serow 5) Specific living style and placentation of the Sloth and Proboscis monkey. Hydatidiform mole in chimpanzee Placentation in different living elephant 6) Similarities of placental structures between human and great apes. Manatee and hyrax 7) Similarities of placental forms in elephants, manatees and rock hyrax with different living styles. Specific placental findings of Himalayan people 8) Specialities of placental pathology in Himalayan mountain people. Conclusions: It was taught that every mammalian species held on placental forms applied to different environ- mental life for their infants, even though their gestational lengths were different. 1. Introduction of effective chemotherapeutic agents. In 1959, I was fortunate tore- ceive an invitation from Prof. Kurt Benirschke at the Boston Lying-in Last October, Scientific American published a special issue about a Hospital. Before that, I had written to Prof. Arthur T. Hertig, Chairman baby's first organ, the placenta [1]. It is full of surprises and amazing of Pathology, Harvard Medical School, asking to study human tropho- science. -

FASD Effects of Alcohol on a Fetus
EFFECTS OF ALCOHOL ON A FETUS “Of all the substances of abuse (including cocaine, heroin, and marijuana), alcohol produces by far the most serious neurobehavioral effects in the fetus.” —Institute of Medicine Report to Congress, 19961 Prenatal exposure to alcohol can damage a fetus at any time, causing problems that persist throughout the individual’s life. There is no known safe level of alcohol use in pregnancy. WHAT IS THE SCOPE OF THE PROBLEM? identified in virtually every part of the body, including the brain, face, eyes, ears, heart, kidneys, and bones. No Alcohol is one of the most dangerous teratogens, which single mechanism can account for all the problems that are substances that can damage a developing fetus.1 Every alcohol causes. Rather, alcohol sets in motion many time a pregnant woman has a drink, her unborn child processes at different sites in the developing fetus: has one, too. Alcohol, like carbon monoxide from cigarettes, passes easily through the placenta from the • Alcohol can trigger cell death in a number of ways, mother's bloodstream into her baby's blood (See Figure causing different parts of the fetus to develop abnormally. 1)—and puts her fetus at risk of having a fetal alcohol • Alcohol can disrupt the way nerve cells develop, travel spectrum disorder (FASD). The blood alcohol level to form different parts of the brain, and function. (BAC) of the fetus becomes equal to or greater than the blood alcohol level of the mother. Because the fetus • By constricting the blood vessels, alcohol interferes with cannot break down alcohol the way an adult can, its BAC blood flow in the placenta, which hinders the delivery 2 remains high for a longer period of time. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

The Placenta
The placenta Learning module Developed by Carolyn Hammer Edited by Fabien Giroux Diagrams By Dr Julien Yockell Lelievre where indicated The placenta – Learning module Table of content 1) Introduction……………………………………………………………………….3 2) Anatomy and Physiology………………………………………………………..6 3) Roles and Functions……………………………………………………………17 4) Development and formation…………………………………..…………….…27 5) What happens after birth……………………………………………….……...34 6) What happens when things go wrong.……………………………………….36 7) Interesting facts about pregnancy………………….…………………………46 2 The placenta – Learning module Introduction 3 The placenta – Learning module What is the placenta? •The placenta is a “vascular (supplied with blood vessels) organ in most mammals that unites the fetus to the uterus of the mother. It mediates the metabolic exchanges of the developing individual through an intimate association of embryonic tissues and of certain uterine tissues, serving the functions of nutrition, respiration, and excretion.” (Online Britannica encyclopaedia) •As the fetus is in full development, it requires a certain amount of gases and nutrients to help support its growth. Because the fetus is unable to do so on its own, the placenta provides these gases and nutrients throughout pregnancy. http://health.allrefer.com/health/plac enta-abruptio-placenta.html 4 The placenta – Learning module What are the main roles of the placenta? •The placenta provides the connection between fetus and mother in order to help carry out many different functions that the growing baby is incapable to do so alone. During pregnancy, the placenta has 6 main roles to maintain good health and a good environment for the growing child: •Respiration •Nutrition •Excretion •Protection •Endocrine •Immunity 5 The placenta – Learning module Anatomy and physiology 6 The placenta – Learning module Structure •A placenta is an organ of round or oval shape that is relatively flat. -

Fetal Descending Aorta/Umbilical Artery Flow Velocity Ratio in Normal Pregnancy at 36-40 Weeks of Gestational Age Riyadh W Alessawi1
American Journal of BioMedicine AJBM 2015; 3(10):674 - 685 doi:10.18081/2333-5106/015-10/674-685 Fetal descending aorta/umbilical artery flow velocity ratio in normal pregnancy at 36-40 Weeks of gestational age Riyadh W Alessawi1 Abstract Doppler velocimetry studies of placental and aortic circulation have gained a wide popularity as it can provide important information regarding fetal well-being and could be used to identify fetuses at risk of morbidity and mortality, thus providing an opportunity to improve fetal outcomes. Prospective longitudinal study conducted through the period from September 2011–July 2012, 125 women with normal pregnancy and uncomplicated fetal outcomes were recruited and subjected to Doppler velocimetry at different gestational ages, from 36 to 40 weeks. Of those, 15 women did not fulfill the protocol inclusion criteria and were not included. In the remaining 110 participants a follow up study of Fetal Doppler velocimetry of Ao and UA was performed at 36 – 40 weeks of gestation. Ao/UA RI: 1.48±0.26, 1.33±0.25, 1.37± 0.20, 1.28±0.07 and 1.39±0.45 respectively and the 95% confidence interval of the mean for five weeks 1.13-1.63. Ao/UA PI: 2.83±2.6, 1.94±0.82, 2.08±0.53, 1.81± 0.12 and 3.28±2.24 respectively. Ao/UA S/D: 2.14±0.72, 2.15±1.14, 1.75±0.61, 2.52±0.18 and 2.26±0.95. The data concluded that a nomogram of descending aorto-placental ratio Ao/UA, S/D, PI and RI of Iraqi obstetric population was established. -
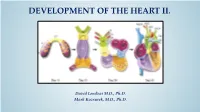
Development of Right Ventricle
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta.