Messages from the Placentae Across Multiple Species a 50 Years
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

Neonate Germinal Stage Blastocyst Embryonic Disk Trophoblast Umbilical Cord Placenta Embryonic Stage Cephalocaudal Proximodistal
neonate umbilical cord Chapter 3 Chapter 3 germinal stage placenta Chapter 3 Chapter 3 blastocyst embryonic stage Chapter 3 Chapter 3 embryonic disk cephalocaudal Chapter 3 Chapter 3 trophoblast proximodistal Chapter 3 Chapter 3 A tube that connects the fetus to the placenta. A newborn baby. Chapter 3 Chapter 3 An organ connected to the uterine wall and to the fetus by the umbilical cord. The placenta The period of development between conception serves as a filter between mother and fetus for and the implantation of the embryo. the exchange of nutrients and wastes. Chapter 3 Chapter 3 The stage of prenatal development that lasts A stage within the germinal period of prenatal from implantation through the eighth week of development in which the zygote has the form pregnancy; it is characterized by the of a sphere of cells surrounding a cavity of fluid. development of the major organ systems. Chapter 3 Chapter 3 The platelike inner part of the blastocyst that From head to tail. differentiates into the ectoderm, mesoderm, and endoderm of the embryo. Chapter 3 Chapter 3 The outer part of the blastocyst from which the From the inner part (or axis) of the body amniotic sac, placenta, and umbilical cord outward. develop. Chapter 3 Chapter 3 ectoderm amniotic sac Chapter 3 Chapter 3 neural tube amniotic fluid Chapter 3 Chapter 3 endoderm fetal stage Chapter 3 Chapter 3 mesoderm stillbirth Chapter 3 Chapter 3 androgens teratogens Chapter 3 Chapter 3 The outermost cell layer of the newly formed The sac containing the fetus. embryo from which the skin and nervous system develop. -

Human Pluripotent Stem Cells As a Model of Trophoblast Differentiation in Both Normal Development and Disease
Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease Mariko Horiia,b,1, Yingchun Lia,b,1, Anna K. Wakelanda,b,1, Donald P. Pizzoa, Katharine K. Nelsona,b, Karen Sabatinib,c, Louise Chang Laurentb,c, Ying Liud,e,f, and Mana M. Parasta,b,2 aDepartment of Pathology, University of California, San Diego, La Jolla, CA 92093; bSanford Consortium for Regenerative Medicine, University of California, San Diego, La Jolla, CA 92093; cDepartment of Reproductive Medicine, University of California, San Diego, La Jolla, CA 92093; dDepartment of Neurosurgery, Center for Stem Cell and Regenerative Medicine, University of Texas Health Sciences Center, Houston, TX 77030; eThe Senator Lloyd and B. A. Bentsen Center for Stroke Research, University of Texas Health Sciences Center, Houston, TX 77030; and fThe Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Sciences Center, Houston, TX 77030 Edited by R. Michael Roberts, University of Missouri–Columbia, Columbia, MO, and approved May 25, 2016 (received for review March 24, 2016) Trophoblast is the primary epithelial cell type in the placenta, a Elf5 (Ets domain transcription factor) and Eomes (Eomeso- transient organ required for proper fetal growth and develop- dermin), also have been shown to be required for maintenance of ment. Different trophoblast subtypes are responsible for gas/nutrient the TSC fate in the mouse (8, 9). exchange (syncytiotrophoblasts, STBs) and invasion and maternal Significantly less is known about TE specification and the TSC vascular remodeling (extravillous trophoblasts, EVTs). Studies of niche in the human embryo (10, 11). -

FASD Effects of Alcohol on a Fetus
EFFECTS OF ALCOHOL ON A FETUS “Of all the substances of abuse (including cocaine, heroin, and marijuana), alcohol produces by far the most serious neurobehavioral effects in the fetus.” —Institute of Medicine Report to Congress, 19961 Prenatal exposure to alcohol can damage a fetus at any time, causing problems that persist throughout the individual’s life. There is no known safe level of alcohol use in pregnancy. WHAT IS THE SCOPE OF THE PROBLEM? identified in virtually every part of the body, including the brain, face, eyes, ears, heart, kidneys, and bones. No Alcohol is one of the most dangerous teratogens, which single mechanism can account for all the problems that are substances that can damage a developing fetus.1 Every alcohol causes. Rather, alcohol sets in motion many time a pregnant woman has a drink, her unborn child processes at different sites in the developing fetus: has one, too. Alcohol, like carbon monoxide from cigarettes, passes easily through the placenta from the • Alcohol can trigger cell death in a number of ways, mother's bloodstream into her baby's blood (See Figure causing different parts of the fetus to develop abnormally. 1)—and puts her fetus at risk of having a fetal alcohol • Alcohol can disrupt the way nerve cells develop, travel spectrum disorder (FASD). The blood alcohol level to form different parts of the brain, and function. (BAC) of the fetus becomes equal to or greater than the blood alcohol level of the mother. Because the fetus • By constricting the blood vessels, alcohol interferes with cannot break down alcohol the way an adult can, its BAC blood flow in the placenta, which hinders the delivery 2 remains high for a longer period of time. -

Self-Organized Amniogenesis by Human Pluripotent Stem Cells in a Biomimetic Implantation-Like Niche
LETTERS PUBLISHED ONLINE: 12 DECEMBER 2016 | DOI: 10.1038/NMAT4829 Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche Yue Shao1†, Kenichiro Taniguchi2†, Katherine Gurdziel3, Ryan F. Townshend2, Xufeng Xue1, Koh Meng Aw Yong1, Jianming Sang1, Jason R. Spence2, Deborah L. Gumucio2* and Jianping Fu1,2,4* Amniogenesis—the development of amnion—is a critical factors seen in the in vivo amniogenic niche: a three-dimensional developmental milestone for early human embryogenesis (3D) extracellular matrix (ECM) that is provided by the basement and successful pregnancy1,2. However, human amniogenesis membrane surrounding the epiblast during implantation11; and a is poorly understood due to limited accessibility to peri- soft tissue bed provided by the uterine wall and trophoblast to implantation embryos and a lack of in vitro models. Here support the developing amnion (Fig. 1a,b). Since amniogenesis ini- we report an ecient biomaterial system to generate human tiates from the expanding pluripotent epiblast, we utilized mTeSR1 amnion-like tissue in vitro through self-organized development medium and basement membrane matrix (Geltrex) to render the of human pluripotent stem cells (hPSCs) in a bioengineered culture permissive for pluripotency maintenance. niche mimicking the in vivo implantation environment. We In this culture system, H9 human embryonic stem cells (hESCs) show that biophysical niche factors act as a switch to toggle were plated as single cells at 30,000 cells cm−2 onto a thick, hPSC self-renewal versus amniogenesis under self-renewal- soft gel bed of Geltrex (with thickness ≥100 µm, bulk Young's permissive biochemical conditions. We identify a unique modulus ∼900 Pa, coated on a glass coverslip), in mTeSR1 medium molecular signature of hPSC-derived amnion-like cells and supplemented with the ROCK inhibitor Y27632 (Fig. -

Prenatal Exposure to Nitrogen Oxides and Its Association with Birth Weight in a Cohort of Mexican Newborns from Morelos, Mexico
Mendoza-Ramirez J, et al. Prenatal Exposure to Nitrogen Oxides and its Association with Birth Weight in a Cohort of Mexican Newborns from Morelos, Mexico. Annals of Global Health. 2018; 84(2), pp. 274–280. DOI: https://doi.org/10.29024/aogh.914 ORIGINAL RESEARCH Prenatal Exposure to Nitrogen Oxides and its Association with Birth Weight in a Cohort of Mexican Newborns from Morelos, Mexico Jessica Mendoza-Ramirez*, Albino Barraza-Villarreal*, Leticia Hernandez-Cadena*, Octavio Hinojosa de la Garza‡,§, José Luis Texcalac Sangrador*, Luisa Elvira Torres- Sanchez*, Marlene Cortez-Lugo*, Consuelo Escamilla-Nuñez*, Luz Helena Sanin-Aguirre† and Isabelle Romieu* Background: The Child-Mother binomial is potentially susceptible to the toxic effects of pollutants because some chemicals interfere with placental transfer of nutrients, thus affecting fetal development, and create an increased the risk of low birth weight, prematurity and intrauterine growth restriction. Objective: To evaluate the impact of prenatal exposure to nitrogen oxides (NOx) on birth weight in a cohort of Mexican newborns. Methodology: We included 745 mother-child pair participants of the POSGRAD cohort study. Information on socio-demographic characteristics, obstetric history, health history and environmental exposure dur- ing pregnancy were readily available and the newborns’ anthropometric measurements were obtained at delivery. Prenatal NOx exposure assessment was evaluated using a Land-Use Regression predictive models considering local monitoring from 60 sites on the State of Morelos. The association between prenatal exposure to NOx and birth weight was estimated using a multivariate linear regression models. Results: The average birth weight was 3217 ± 439 g and the mean of NOx concentration was 21 ppb (Interquartile range, IQR = 6.95 ppb). -

The Science of Amnioexcite™ Three Layer Placental Membrane Allograft
The Science of AmnioExcite™ Three Layer Placental Membrane Allograft REV. 10-2020 The Science of AmnioExcite™ Placental Membrane Allograft AmnioExcite™ is a full-thickness decellularized placental membrane. AmnioExcite™ is a lyophilized, full-thickness placental membrane allograft decellularized with LifeNet Health’s proprietary Matracell® process and patent pending technology and intended for homologous use as a barrier membrane.(1) Inclusion of the intact amniotic and chorionic membranes, as well as the trophoblast layer, makes it thicker than most available amniotic-only or amniotic-chorionic allografts, and provides a robust protective covering while also delivering superior handling. AmnioExcite™ retains the placental membrane’s naturally occurring growth factors, cytokines, protease inhibitors, and extracellular matrix components, such as proteoglycans, collagen and fibronectin(2) In vitro studies have shown that these endogenous factors are capable of inducing cellular proliferation and migration, mitigating inflammation, and inhibiting protein degradation(3-5) STRUCTURE OF THE THREE LAYER PLACENTAL MEMBRANE AMNIOTIC MEMBRANE CHORIONIC MEMBRANE TROPHOBLAST LAYER The placental membrane is comprised of the amnion and chorion (6). The amnion, also called amniotic membrane (AM) has five layers, including the epithelium, basement membrane, compact layer, fibroblast layer, and the spongy layer(6), which provide important extracellular membrane components, as well as a wide variety of growth factors, cytokines, and other proteins.(7) While these characteristics are important, the AM by itself lacks substantial structure for providing a protective covering and contains only a small portion of the biological factors found in the full-thickness placental membrane. AM-only grafts can also be difficult to apply and may migrate away from the intended site of application.(8) The chorion is comprised of four layers, including the cellular layer, reticular layer, the pseudobasement membrane and the trophoblast layer (TL) (6). -
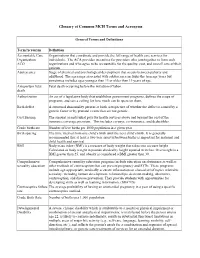
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

Systematic Reviews Ajog.Org
Systematic Reviews ajog.org The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis Ste´phanie Roberge, PhD; Kypros Nicolaides, MD; Suzanne Demers, MD, MSc; Jon Hyett, MD; Nils Chaillet, PhD; Emmanuel Bujold, MD, MSc BACKGROUND: Preeclampsia and fetal growth restriction are major causes of perinatal death and handicap in survivors. Randomized clinical trials have reported that the risk of preeclampsia, severe preeclampsia, and fetal growth restriction can be reduced by the prophylactic use of aspirin in high-risk women, but the appropriate dose of the drug to achieve this objective is not certain. OBJECTIVE: We sought to estimate the impact of aspirin dosage on the prevention of preeclampsia, severe preeclampsia, and fetal growth restriction. STUDY DESIGN: We performed a systematic review and meta-analysis of randomized controlled trials comparing the effect of daily aspirin or placebo (or no treatment) during pregnancy. We searched MEDLINE, Embase, Web of Science, and Cochrane Central Register of Controlled Trials up to December 2015, and study bibliographies were reviewed. Authors were contacted to obtain additional data when needed. Relative risks for preeclampsia, severe preeclampsia, and fetal growth restriction were calculated with 95% confidence intervals using random-effect models. Dose-response effect was evaluated using meta-regression and reported as adjusted R2. Analyses were stratified according to gestational age at initiation of aspirin (16 and >16 weeks) and repeated after exclusion of studies at high risk of biases. RESULTS: In all, 45 randomized controlled trials included a total of 20,909 pregnant women randomized to between 50-150 mg of aspirin daily. -

What Is the Role of the Placenta—Does It Protect Against Or Is It a Target for Insult?
Teratology Primer, 3rd Edition www.teratology.org/primer What Is the Role of the Placenta—Does It Protect Against or Is It a Target for Insult? Richard K. Miller University of Rochester School of Medicine & Dentistry Rochester, New York The placenta is not just a barrier but has many functions that are vital to the health of the embryo/fetus. The placenta is the anchor, the conduit, and the controller of pregnancy—and it can also be a target for toxicant action. The placenta encompasses not only the chorioallantoic placenta but all of its extraembryonic membranes (chorion/amnion) and the yolk sac. (Figure 1). The placenta and its membranes secure the embryo and fetus to the decidua (uterine lining) and release a variety of steroid and protein hormones that characterize the physiology of the pregnant female. Figure 1. Placental Structure in the Mouse. Figures depict early development of the mouse conceptus at embryonic days (E3.5, E7.5, E12.5). In the fetus, the visceral yolk sac (vYS) inverts and remains active throughout the entire gestation providing for transfer of selective large molecules, e.g., immunoglobulins IgG and vitamin B12. Abbreviations: Al, allantois; Am, amnion; Ch, chorion; Dec, decidua; Emb, embryo; Epc, ectoplacental cone; ICM, inner cell mass; Lab, Labyrinth; pYS, parietal yolk sac; SpT, spongiotrophoblast; TCG, trophoblast giant cell; Umb Cord, umbilical cord; vYS, visceral yolk sac; C-TGC, maternal blood canal trophoblast giant cell; P-TGC, parietal trophoblast giant cell; S-TGC, sinusoidal trophoblast giant cell; SpA-TGC, Spiral artery-associated trophoblast giant cell; Cyan-trophectoderm and trophoblast lineage, Black- inner cell mass and embryonic ectoderm; Gray -endoderm, Red-maternal vasculature, Purple-mesoderm, Yellow- decidua, Pink-fetal blood vessels in labyrinth. -

Equine Placenta – Marvelous Organ and a Lethal Weapon
Equine placenta – marvelous organ and a lethal weapon Malgorzata Pozor, DVM, PhD, Diplomate ACT Introduction Placenta has been defined as: „an apposition between parent (usually maternal) and fetal tissue in order to establish physiological exchange” (1). Another definition of this important organ was proposed by Steven and Morris: „a device consisting of one or more transport epithelia located between fetal and maternal blood supply” (2). The main function of placenta is to provide an interface between the dam and the the fetus and to allow the metabolic exchange of the the nutrients, oxygen and waste material. The maternal circulation is brought into a close apposition to the fetal circulation, while a separation of these two circulatory systems remain separated (3). A degree and complexity of this „intimate relationship” varies greately between species mostly due to the structural diversity of the extraembryonic membranes of the vertebrates. The early feto-maternal exchange in the equine pregnancy is established as early as on day 22 after fertilization. The fetal and choriovitellin circulations are already present, the capsule ruptures and the allantois is already visible (4). The allantois starts expanding by day 32 and vascularizes approximately 90% of the chorion and fuses with it to form chorioallantois by day 38 of gestation (5). The equine placenta continues increasing its complexity till approximately day 150 of gestation. Equids have epitheliochorial placenta, there are six leyers separating maternal and fetal circulation, and there are no erosion of the luminal, maternal epithelium, like in ruminants (6). Thousands of small chorionic microvilli develop and penetrate into endometrial invaginations. -

From Trophoblast to Human Placenta
From Trophoblast to Human Placenta (from The Encyclopedia of Reproduction) Harvey J. Kliman, M.D., Ph.D. Yale University School of Medicine I. Introduction II. Formation of the placenta III. Structure and function of the placenta IV. Complications of pregnancy related to trophoblasts and the placenta Glossary amnion the inner layer of the external membranes in direct contact with the amnionic fluid. chorion the outer layer of the external membranes composed of trophoblasts and extracellular matrix in direct contact with the uterus. chorionic plate the connective tissue that separates the amnionic fluid from the maternal blood on the fetal surface of the placenta. chorionic villous the final ramification of the fetal circulation within the placenta. cytotrophoblast a mononuclear cell which is the precursor cell of all other trophoblasts. decidua the transformed endometrium of pregnancy intervillous space the space in between the chorionic villi where the maternal blood circulates within the placenta invasive trophoblast the population of trophoblasts that leave the placenta, infiltrates the endo– and myometrium and penetrates the maternal spiral arteries, transforming them into low capacitance blood channels. Sunday, October 29, 2006 Page 1 of 19 From Trophoblasts to Human Placenta Harvey Kliman junctional trophoblast the specialized trophoblast that keep the placenta and external membranes attached to the uterus. spiral arteries the maternal arteries that travel through the myo– and endometrium which deliver blood to the placenta. syncytiotrophoblast the multinucleated trophoblast that forms the outer layer of the chorionic villi responsible for nutrient exchange and hormone production. I. Introduction The precursor cells of the human placenta—the trophoblasts—first appear four days after fertilization as the outer layer of cells of the blastocyst.