Neuroimaging and Neuromodulation: Complementary Approaches for Identifying the Neuronal Correlates of Tinnitus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Eloreta Quantitative EEG Study
brain sciences Article Power Spectral Differences between Transient Epileptic and Global Amnesia: An eLORETA Quantitative EEG Study Jacopo Lanzone 1,* , Claudio Imperatori 2 , Giovanni Assenza 1 , Lorenzo Ricci 1 , Benedetto Farina 2, Vincenzo Di Lazzaro 1 and Mario Tombini 1 1 Neurology, Neurophysiology and Neurobiology Unit, Department of Medicine, Università Campus Bio-Medico di Roma, 00128 Rome, Italy; [email protected] (G.A.); [email protected] (L.R.); [email protected] (V.D.L.); [email protected] (M.T.) 2 Cognitive and Clinical Psychology Laboratory, Department of Human Sciences, European University of Rome, Via degli Aldobrandeschi 190, 00163 Rome, Italy; [email protected] (C.I.); [email protected] (B.F.) * Correspondence: [email protected] Received: 14 July 2020; Accepted: 4 September 2020; Published: 6 September 2020 Abstract: Transient epileptic amnesia (TEA) is a rare epileptic condition, often confused with transient global amnesia (TGA). In a real-life scenario, differential diagnosis between these two conditions can be hard. In this study we use power spectral analysis empowered by exact Low Resolution Brain Electromagnetic Tomography (eLORETA) to evidence the differences between TEA and TGA. Fifteen patients affected by TEA (64.2 5.2 y.o.; 11 female/4 male; 10 left and 5 right temporal epileptic focus) ± and 15 patients affected by TGA (65.8 7.2 y.o.; 11 females/4 males) were retrospectively identified in ± our clinical records. All patients recorded EEGs after symptoms offset. EEGs were analyzed with eLORETA to evidence power spectral contrast between the two conditions. We used an inverse problem solution to localize the source of spectral differences. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

The Pre/Parasubiculum: a Hippocampal Hub for Scene- Based Cognition? Marshall a Dalton and Eleanor a Maguire
Available online at www.sciencedirect.com ScienceDirect The pre/parasubiculum: a hippocampal hub for scene- based cognition? Marshall A Dalton and Eleanor A Maguire Internal representations of the world in the form of spatially which posits that one function of the hippocampus is to coherent scenes have been linked with cognitive functions construct internal representations of scenes in the ser- including episodic memory, navigation and imagining the vice of memory, navigation, imagination, decision-mak- future. In human neuroimaging studies, a specific hippocampal ing and a host of other functions [11 ]. Recent inves- subregion, the pre/parasubiculum, is consistently engaged tigations have further refined our understanding of during scene-based cognition. Here we review recent evidence hippocampal involvement in scene-based cognition. to consider why this might be the case. We note that the pre/ Specifically, a portion of the anterior medial hippocam- parasubiculum is a primary target of the parieto-medial pus is consistently engaged by tasks involving scenes temporal processing pathway, it receives integrated [11 ], although it is not yet clear why a specific subre- information from foveal and peripheral visual inputs and it is gion of the hippocampus would be preferentially contiguous with the retrosplenial cortex. We discuss why these recruited in this manner. factors might indicate that the pre/parasubiculum has privileged access to holistic representations of the environment Here we review the extant evidence, drawing largely from and could be neuroanatomically determined to preferentially advances in the understanding of visuospatial processing process scenes. pathways. We propose that the anterior medial portion of the hippocampus represents an important hub of an Address extended network that underlies scene-related cognition, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, and we generate specific hypotheses concerning the University College London, 12 Queen Square, London WC1N 3BG, UK functional contributions of hippocampal subfields. -

The Surgical Prognostic Significance of the Electroencephalographic Prediction of Ammon's Horn Sclerosis in Epileptics by W
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.21.1.24 on 1 February 1958. Downloaded from J. Neurol. Neurosurg. Psychiat., 1958, 21, 24. THE SURGICAL PROGNOSTIC SIGNIFICANCE OF THE ELECTROENCEPHALOGRAPHIC PREDICTION OF AMMON'S HORN SCLEROSIS IN EPILEPTICS BY W. A. KENNEDY and DENIS HILL BASED ON PATHOLOGICAL MATERIAL SUPPLIED BY J. B. CAVANAGH and A. MEYER From the Institute ofPsychiatry and the Guy's-Maudsley Neurosurgical Unit, London In a previous communication (Falconer, Hill, Jasper (1954) and recently by Rasmussen (1957). Meyer, Mitchell, and Pond, 1955) the results of The diagnostic and pathological data and the treating intractable temporal lobe epilepsy by tem- therapeutic effects of temporal lobectomy carried poral lobectomy were given. A survey was reported out on a larger series of 50 cases are now available of the clinical, electroencephalographic, radiological, and a preliminary report has been given (Falconer, and pathological findings in 31 cases. The follow-up Meyer, Hill, and Wilson, 1957). Following Earle etguest. Protected by copyright. study of the patients showed beneficial effects upon al. (1953) the pathological findings have been classi- the epilepsy and upon the personality disorder in a fied in two main groups. While all cases in which a proportion which compared very favourably with space-occupying lesion could be anticipated on most published series. The opinion was expressed clinical, radiological, or electroencephalographic that this was due to inclusion of the uncus, Ammon's grounds were excluded from the series, there were, horn, and possibly the amygdaloid nucleus in the nevertheless, 14 cases in which very small focal resected tissue. -

Subtemporal Transparahippocampal Amygdalohippocampectomy for Surgical Treatment of Mesial Temporal Lobe Epilepsy Technical Note
Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy Technical note T. S. Park, M.D., Blaise F. D. Bourgeois, M.D., Daniel L. Silbergeld, M.D., and W. Edwin Dodson, M.D. Department of Neurology and Neurological Surgery, Washington University School of Medicine, and St. Louis Children's Hospital, St. Louis, Missouri Amygdalohippocampectomy (AH) is an accepted surgical option for treatment of medically refractory mesial temporal lobe epilepsy. Operative approaches to the amygdala and hippocampus that previously have been reported include: the sylvian fissure, the superior temporal sulcus, the middle temporal gyrus, and the fusiform gyrus. Regardless of the approach, AH permits not only extirpation of an epileptogenic focus in the amygdala and anterior hippocampus, but interruption of pathways of seizure spread via the entorhinal cortex and the parahippocampal gyrus. The authors report a modification of a surgical technique for AH via the parahippocampal gyrus, in which excision is limited to the anterior hippocampus, amygdala and parahippocampal gyrus while preserving the fusiform gyrus and the rest of the temporal lobe. Because transparahippocampal AH avoids injury to the fusiform gyrus and the lateral temporal lobe, it can be performed without intracarotid sodium amobarbital testing of language dominance and language mapping. Thus the operation would be particularly suitable for pediatric patients in whom intraoperative language mapping before resection is difficult. Key Words * amygdalohippocampectomy * complex partial seizure * parahippocampal gyrus * subtemporal approach Currently several different variations of temporal lobe resections are used for medically intractable complex partial seizures.[4,6,8,18,21,30,34] Among these operations is amygdalohippocampectomy (AH), first described in 1958 by Niemeyer,[16] who approached the amygdala and hippocampus through an incision on the middle temporal gyrus. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -
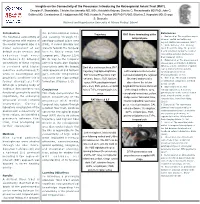
Insights on the Connectivity of the Precuneus: Introducing the Retrosplenial Aslant Tract (RAT)
Insights on the Connectivity of the Precuneus: Introducing the Retrosplenial Aslant Tract (RAT). Georgios P. Skandalakis; Christos Koutsarnakis MD, MSc; Aristotelis Kalyvas; Dimitris G. Placantonakis MD PhD; John G. Golfinos MD; Constantinos G. Hadjipanayis MD PhD; Kostas N. Fountas MD PhD FAANS; Eftychia Z. Kapsalaki MD; George S. Stranjalis National and Kapodistrian University of Athens Medical School Introduction the parieto-occipital sulcus, References Trajectory RAT fibers terminating at the The functional connectivity of and passing through the 1. Epstein et al. The cognitive map in temporal pole humans: spatial navigation and the precuneus with regions of parahippocampal place area beyond. Nature neuroscience. (2017) the medial temporal lobe is a (PPA), it curves laterally and 2. Wade-Bohleber et al. Thinking major component of our projects towards the temporal about the past to shape the present: default mode network and horn to finally reach the Neural activation during the recall of relationship episodes. Behavioural underlies high order temporal pole. (Figures 1,3,4) Brain Research (2018). functions.(1-5) Stronger On its way to the temporal 3. Hebscher et al. The precuneus and connectivity of these regions pole this tracts also displays hippocampus contribute to individual differences in the unfolding of spatial is corelated with higher connections with the lingual, Dark blue continuous lines, RAT representations during episodic cognitive performances(7) parahippocampal and fusiform trajectory; highlighted light blue, Left hemisphere infero-medial autobiographical memory. while in neurological and gyri, inferior longitudinal RAT Cortical Projections; CaF, view demonstrating the regional Neuropsychologia. (2018) psychiatric conditions this is fasciculus and hippocampal calcarine fissure; FuG, fusiform fiber tract anatomy after 4. -

Surgery of the Amygdala and Uncus: a Case Series of Glioneuronal Tumors
Acta Neurochirurgica (2020) 162:795–801 https://doi.org/10.1007/s00701-020-04249-1 TECHNICAL NOTE - TUMOR - GLIOMA Surgery of the amygdala and uncus: a case series of glioneuronal tumors Andrew C. Vivas1 & Stephen Reintjes1 & Nir Shimony 2,3,4 & Fernando L. Vale5 Received: 5 November 2019 /Accepted: 23 January 2020 /Published online: 30 January 2020 # The Author(s) 2020 Abstract Background Patients with a lesion within the amygdala and uncus may develop temporal lobe epilepsy despite having functional mesial structures. Resection of functional hippocampus and surrounding structures may lead to unacceptable iatrogenic deficits. To our knowledge, there is limited descriptions of surgical techniques for selectively resecting the amygdala and uncus lesions while preserving the hippocampus in patients with language-dominant temporal lobe pathology. Methods Thirteen patients with language-dominant temporal lobe epilepsy related to amygdala-centric lesions were identified. Patients with sclerosis of the mesial structures or evidence of pathology outside of the amygdala-uncus region were excluded. Neuropsychological evaluation confirmed normal function of the mesial structures ipsilateral to the lesion. All patients were worked up with video-EEG, high-resolution brain MRI, neuro-psychology evaluation, and either Wada or functional MRI testing. Results All patients underwent selective resection of the lesion including amygdala and uncus with preservation of the hippo- campus via a transcortical inferior temporal gyrus approach to the mesial temporal lobe. Pathology was compatible with glioneuronal tumors. Post-operative MRI demonstrated complete resection in all patients. Eight of the thirteen patients underwent post-operative neuropsychology evaluations and did not demonstrate any significant decline in tasks of delayed verbal recall or visual memory based on the Rey Auditory Verbal Learning Test (RAVLT). -

Sheep Brain Dissection Guide
Sheep Brain Dissection Guide DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the three- dimensional structure of the brain and teach you one of the great methods of studying the brain: looking at its structure. One of the great truths of studying biology is the saying that "anatomy precedes physiology". You will get sick of me saying that phrase this phrase if I teach well. What this phrase means is that how something is put together tells us much about how it works. My challenge to you with this exercise and throughout the term will be to examine a structure and think what this means about the operation of the brain. Your ideas can be as valid as anyone else's who has tackled this delightfully impossible task if you think carefully While the course will emphasize the human brain, observation and evolution indicate that there are many similarities between the sheep brain and the human brain. Even the differences are instructive and help us to learn about the brain. Being able to locate important structures in the sheep brain will be of great benefit to understanding how structures are related to each other in the human brain. If the same structure exists in both brains (and most structures are the same), they are in the same relative location. During the course of the dissection, I will point out some of the differences between brains so that you will be better able to appreciate the development of the human brain. -

Anatomic Moment Limbic System Anatomy: an Overview
Anatomic Moment Limbic System Anatomy: An Overview Leighton P. Mark,1.3 David L. Daniels, 1 Thomas P. Naidich,2 and Jessica A. Borne1 The anatomy of the limbic system has become shaped sulcus (Fig. 2) designated as 1) the hip more relevant due to the development of high pocampal fissure where it lies superior to the resolution and functional magnetic resonance parahippocampal gyrus, and 2) the callosal sulcus (MR) imaging. This review is the first of a series where it lies inferior to the cingulate gyrus of Anatomic Moments designed to highlight se (Fig. 3). lected features of limbic system anatomy in order Nested within this )-shaped sulcus is another ) to facilitate their application to MR interpretation. shaped structure formed by 1) the dentate gyrus Limbic system terminology of early anatomists and hippocampus in the temporal lobe (Figs. 2 was largely descriptive (Table 1), but the nomen and 4), 2) the hippocampal tail which consists of clature used in this series will follow that of recent thin gray and white matter structures located just authors (Table 2) (1-7). posterior and inferior to the splenium of the In accordance with the curvilinear development corpus callosum (Fig. 5), and 3) the supracallosal of the cerebral hemispheres (Fig. 1), the struc gyrus which is located inferior to the callosal tures of the limbic lobe form a series of "nested" sulcus. The supracallosal gyrus is intimately ap )-shaped curves (Fig. 2). plied to the upper surface of the corpus callosum, The widest curve is formed by the large limbic and contains gray matter termed the indusium gyrus which is designated as 1) the parahippo griseum (Fig. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

Topographically Specific Hippocampal Projections Target Functionally Distinct Prefrontal Areas in the Rhesus Monkey
HIPPOCAMPUS 5:511-533 (1995) Topographically Specific Hippocampal Projections Target Functionally Distinct Prefrontal Areas in the Rhesus Monkey Helen Barbas1r2and Gene J. Blatt2 [Department of Health Sciences, Boston University and lJ2Departmentof Anatomy and Neurobiology, Boston University School of Medicine, Boston, Massachusetts ABSTRACT: The sources of ipsilateral projections from the hippocam- KEY WORDS: medial prefrontal cortex, orbitofrontal pal formation, the presubiculum, area 29a-c, and parasubiculum to me- cortex, memory, CA1, subiculum, .presubiculum, area dial, orbital, and lateral prefrontal cortices were studied with retrograde 29, lateral prefrontal cortex, working memory tracers in 27 rhesus monkeys. labeled neurons within the hippocampal formation (CA1, CA1’, prosubiculum, and subiculum) were found ros- trally, although some were noted throughout the entire rostrocaudal ex- tent of the hippocampal formation. Most labeled neurons in the hip- pocampal formation projected to medial prefrontal cortices, followed by orbital areas. In addition, there were differences in the topography of af- ferent neurons projecting to medial when compared with orbital cortices. Labeled neurons innervating medial cortices were found mainly in the The prefrontal cortex in the rhesus monkey is a large CA1’ and CA1 fields rostrally, but originated in the subicular fields cau- cortical cxpanse associated with complex cognitive, dally. In contrast, labeled neurons which innervated orbital cortices were mnemonic, and emotional processes (for rcvicws, see considerably more focal, emanating from the same relative position within Fuster, 1989; Barbas, 1995a). The functions of pre- a field throughout the rostrocaudal extent of the hippocampal formation. frontal areas are likely to depend on their connections In marked contrast to the pattern of projection to medial and orbital prefrontal cortices, lateral prefrontal areas received projections from only with other cortical and subcortical structures.