Anatomical Variations of the Temporomesial Structures In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Eloreta Quantitative EEG Study
brain sciences Article Power Spectral Differences between Transient Epileptic and Global Amnesia: An eLORETA Quantitative EEG Study Jacopo Lanzone 1,* , Claudio Imperatori 2 , Giovanni Assenza 1 , Lorenzo Ricci 1 , Benedetto Farina 2, Vincenzo Di Lazzaro 1 and Mario Tombini 1 1 Neurology, Neurophysiology and Neurobiology Unit, Department of Medicine, Università Campus Bio-Medico di Roma, 00128 Rome, Italy; [email protected] (G.A.); [email protected] (L.R.); [email protected] (V.D.L.); [email protected] (M.T.) 2 Cognitive and Clinical Psychology Laboratory, Department of Human Sciences, European University of Rome, Via degli Aldobrandeschi 190, 00163 Rome, Italy; [email protected] (C.I.); [email protected] (B.F.) * Correspondence: [email protected] Received: 14 July 2020; Accepted: 4 September 2020; Published: 6 September 2020 Abstract: Transient epileptic amnesia (TEA) is a rare epileptic condition, often confused with transient global amnesia (TGA). In a real-life scenario, differential diagnosis between these two conditions can be hard. In this study we use power spectral analysis empowered by exact Low Resolution Brain Electromagnetic Tomography (eLORETA) to evidence the differences between TEA and TGA. Fifteen patients affected by TEA (64.2 5.2 y.o.; 11 female/4 male; 10 left and 5 right temporal epileptic focus) ± and 15 patients affected by TGA (65.8 7.2 y.o.; 11 females/4 males) were retrospectively identified in ± our clinical records. All patients recorded EEGs after symptoms offset. EEGs were analyzed with eLORETA to evidence power spectral contrast between the two conditions. We used an inverse problem solution to localize the source of spectral differences. -
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

'What' but Not 'Where' Auditory Processing Pathway
NeuroImage 82 (2013) 295–305 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Emotion modulates activity in the ‘what’ but not ‘where’ auditory processing pathway James H. Kryklywy d,e, Ewan A. Macpherson c,f, Steven G. Greening b,e, Derek G.V. Mitchell a,b,d,e,⁎ a Department of Psychiatry, University of Western Ontario, London, Ontario N6A 5A5, Canada b Department of Anatomy & Cell Biology, University of Western Ontario, London, Ontario N6A 5A5, Canada c National Centre for Audiology, University of Western Ontario, London, Ontario N6A 5A5, Canada d Graduate Program in Neuroscience, University of Western Ontario, London, Ontario N6A 5A5, Canada e Brain and Mind Institute, University of Western Ontario, London, Ontario N6A 5A5, Canada f School of Communication Sciences and Disorders, University of Western Ontario, London, Ontario N6A 5A5, Canada article info abstract Article history: Auditory cortices can be separated into dissociable processing pathways similar to those observed in the vi- Accepted 8 May 2013 sual domain. Emotional stimuli elicit enhanced neural activation within sensory cortices when compared to Available online 24 May 2013 neutral stimuli. This effect is particularly notable in the ventral visual stream. Little is known, however, about how emotion interacts with dorsal processing streams, and essentially nothing is known about the impact of Keywords: emotion on auditory stimulus localization. In the current study, we used fMRI in concert with individualized Auditory localization Emotion auditory virtual environments to investigate the effect of emotion during an auditory stimulus localization fi Auditory processing pathways task. Surprisingly, participants were signi cantly slower to localize emotional relative to neutral sounds. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -
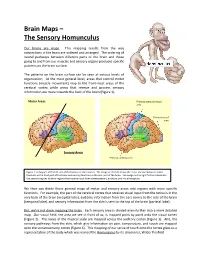
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Subdivisions of Auditory Cortex and Processing Streams in Primates
Colloquium Subdivisions of auditory cortex and processing streams in primates Jon H. Kaas*† and Troy A. Hackett‡ Departments of †Psychology and ‡Hearing and Speech Sciences, Vanderbilt University, Nashville, TN 37240 The auditory system of monkeys includes a large number of histochemical studies in chimpanzees and humans, and nonin- interconnected subcortical nuclei and cortical areas. At subcortical vasive functional studies in humans. levels, the structural components of the auditory system of mon- keys resemble those of nonprimates, but the organization at The Core Areas of Auditory Cortex cortical levels is different. In monkeys, the ventral nucleus of the Originally, auditory cortex of monkeys was thought to be orga- medial geniculate complex projects in parallel to a core of three nized much as in cats, with a single primary area, AI, in the primary-like auditory areas, AI, R, and RT, constituting the first cortex of the lower bank of the lateral sulcus and a second area, stage of cortical processing. These areas interconnect and project AII, deeper in the sulcus (e.g., ref. 3). This concept fits well with to the homotopic and other locations in the opposite cerebral the early view that auditory, somatosensory, and visual systems hemisphere and to a surrounding array of eight proposed belt all have two fields. However, we now know that primates have areas as a second stage of cortical processing. The belt areas in turn a number of sensory representations for each modality, and project in overlapping patterns to a lateral parabelt region with at several somatosensory and auditory fields can be considered least rostral and caudal subdivisions as a third stage of cortical primary or primary like in character. -

The Pre/Parasubiculum: a Hippocampal Hub for Scene- Based Cognition? Marshall a Dalton and Eleanor a Maguire
Available online at www.sciencedirect.com ScienceDirect The pre/parasubiculum: a hippocampal hub for scene- based cognition? Marshall A Dalton and Eleanor A Maguire Internal representations of the world in the form of spatially which posits that one function of the hippocampus is to coherent scenes have been linked with cognitive functions construct internal representations of scenes in the ser- including episodic memory, navigation and imagining the vice of memory, navigation, imagination, decision-mak- future. In human neuroimaging studies, a specific hippocampal ing and a host of other functions [11 ]. Recent inves- subregion, the pre/parasubiculum, is consistently engaged tigations have further refined our understanding of during scene-based cognition. Here we review recent evidence hippocampal involvement in scene-based cognition. to consider why this might be the case. We note that the pre/ Specifically, a portion of the anterior medial hippocam- parasubiculum is a primary target of the parieto-medial pus is consistently engaged by tasks involving scenes temporal processing pathway, it receives integrated [11 ], although it is not yet clear why a specific subre- information from foveal and peripheral visual inputs and it is gion of the hippocampus would be preferentially contiguous with the retrosplenial cortex. We discuss why these recruited in this manner. factors might indicate that the pre/parasubiculum has privileged access to holistic representations of the environment Here we review the extant evidence, drawing largely from and could be neuroanatomically determined to preferentially advances in the understanding of visuospatial processing process scenes. pathways. We propose that the anterior medial portion of the hippocampus represents an important hub of an Address extended network that underlies scene-related cognition, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, and we generate specific hypotheses concerning the University College London, 12 Queen Square, London WC1N 3BG, UK functional contributions of hippocampal subfields. -

The Surgical Prognostic Significance of the Electroencephalographic Prediction of Ammon's Horn Sclerosis in Epileptics by W
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.21.1.24 on 1 February 1958. Downloaded from J. Neurol. Neurosurg. Psychiat., 1958, 21, 24. THE SURGICAL PROGNOSTIC SIGNIFICANCE OF THE ELECTROENCEPHALOGRAPHIC PREDICTION OF AMMON'S HORN SCLEROSIS IN EPILEPTICS BY W. A. KENNEDY and DENIS HILL BASED ON PATHOLOGICAL MATERIAL SUPPLIED BY J. B. CAVANAGH and A. MEYER From the Institute ofPsychiatry and the Guy's-Maudsley Neurosurgical Unit, London In a previous communication (Falconer, Hill, Jasper (1954) and recently by Rasmussen (1957). Meyer, Mitchell, and Pond, 1955) the results of The diagnostic and pathological data and the treating intractable temporal lobe epilepsy by tem- therapeutic effects of temporal lobectomy carried poral lobectomy were given. A survey was reported out on a larger series of 50 cases are now available of the clinical, electroencephalographic, radiological, and a preliminary report has been given (Falconer, and pathological findings in 31 cases. The follow-up Meyer, Hill, and Wilson, 1957). Following Earle etguest. Protected by copyright. study of the patients showed beneficial effects upon al. (1953) the pathological findings have been classi- the epilepsy and upon the personality disorder in a fied in two main groups. While all cases in which a proportion which compared very favourably with space-occupying lesion could be anticipated on most published series. The opinion was expressed clinical, radiological, or electroencephalographic that this was due to inclusion of the uncus, Ammon's grounds were excluded from the series, there were, horn, and possibly the amygdaloid nucleus in the nevertheless, 14 cases in which very small focal resected tissue. -

Human Parietal Cortex in Action Jody C Culham and Kenneth F Valyear
Human parietal cortex in action Jody C Culham and Kenneth F Valyear Experiments using functional neuroimaging and transcranial owes, in large part, to the rapid growth of neuroimaging magnetic stimulation in humans have revealed regions of the studies, particularly experiments using functional mag- parietal lobes that are specialized for particular visuomotor netic resonance imaging (fMRI) and transcranial mag- actions, such as reaching, grasping and eye movements. In netic stimulation (TMS). addition, the human parietal cortex is recruited by processing and perception of action-related information, even when no In one popular view of the visual system [1], visual overt action occurs. Such information can include object shape information is segregated along two pathways: the ventral and orientation, knowledge about how tools are employed and stream (occipito-temporal cortex) computes vision for the understanding of actions made by other individuals. We perception, whereas the dorsal stream (occipito-parietal review the known subregions of the human posterior parietal cortex) computes vision for action. Here, we review cortex and the principles behind their organization. recent advances that address the organization of the posterior parietal cortex and the action-related subregions Addresses within it. We begin by focusing on the role of the dorsal Department of Psychology, Social Science Centre, University of stream in visually-guided real actions. However, we then Western Ontario, London, Ontario, Canada, N6A 5C2 discuss a topic that -

Neuroanatomy: Dissection of the Sheep Brain
Neuroanatomy: Dissection of the Sheep Brain The basic neuroanatomy of the mammalian brain is similar for all species. Instead of using a rodent brain (too small) or a human brain (no volunteer donors), we will study neuroanatomy by examining the brain of the sheep. We will be looking as several structures within the central nervous system (CNS), which is composed of the brain and spinal cord. You will learn individual structures now; later you will be looking at brain systems to see how different parts of the brain form functional units. Just as we differ with respect to individual characteristics, brains also differ slightly in the size, coloration, and shape of some structures. Carefully examine the brain you and your group are dissecting and try to look around the room at some other brains so you can see how the structures may vary. This is especially important when examining the cranial nerves, since rarely will all 12 nerve pairs be preserved on any one brain. This handout will guide you through the dissection and identification of structures. Refer to your text and the accompanying CD for additional information about comparative structures and functions. The last page of this handout lists all the structures you should be prepared to identify for the test. If the structure is marked with *, you should be prepared to identify the function of that structure. Check out the terrific tutorial of a sheep brain dissection on the web site at the University of Scranton (http://academic.uofs.edu/department/psych/sheep/). Lateral View of the Sheep Brain Dorsal Horizontal Section Anterior Posterior Ventral Olfactory bulbs Optic nerves Look for Medulla and chiasm pituitary here External and Midline Anatomy Examine the sheep brain with the membranes intact.