Journal of Neurotherapy: Investigations In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Eloreta Quantitative EEG Study
brain sciences Article Power Spectral Differences between Transient Epileptic and Global Amnesia: An eLORETA Quantitative EEG Study Jacopo Lanzone 1,* , Claudio Imperatori 2 , Giovanni Assenza 1 , Lorenzo Ricci 1 , Benedetto Farina 2, Vincenzo Di Lazzaro 1 and Mario Tombini 1 1 Neurology, Neurophysiology and Neurobiology Unit, Department of Medicine, Università Campus Bio-Medico di Roma, 00128 Rome, Italy; [email protected] (G.A.); [email protected] (L.R.); [email protected] (V.D.L.); [email protected] (M.T.) 2 Cognitive and Clinical Psychology Laboratory, Department of Human Sciences, European University of Rome, Via degli Aldobrandeschi 190, 00163 Rome, Italy; [email protected] (C.I.); [email protected] (B.F.) * Correspondence: [email protected] Received: 14 July 2020; Accepted: 4 September 2020; Published: 6 September 2020 Abstract: Transient epileptic amnesia (TEA) is a rare epileptic condition, often confused with transient global amnesia (TGA). In a real-life scenario, differential diagnosis between these two conditions can be hard. In this study we use power spectral analysis empowered by exact Low Resolution Brain Electromagnetic Tomography (eLORETA) to evidence the differences between TEA and TGA. Fifteen patients affected by TEA (64.2 5.2 y.o.; 11 female/4 male; 10 left and 5 right temporal epileptic focus) ± and 15 patients affected by TGA (65.8 7.2 y.o.; 11 females/4 males) were retrospectively identified in ± our clinical records. All patients recorded EEGs after symptoms offset. EEGs were analyzed with eLORETA to evidence power spectral contrast between the two conditions. We used an inverse problem solution to localize the source of spectral differences. -

Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment
The Journal of Neuroscience, December 1989, 9(12): 4355-4370 Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment Stuart Zola-Morgan,’ Larry Ft. Squire,’ David G. Amaral,2 and Wendy A. Suzuki2J Veterans Administration Medical Center, San Diego, California, 92161, and Department of Psychiatry, University of California, San Diego, La Jolla, California 92093, The Salk Institute, San Diego, California 92136, and 3Group in Neurosciences, University of California, San Diego, La Jolla, California 92093 In monkeys, bilateral damage to the medial temporal region Moss, 1984). (In this notation, H refers to the hippocampus, A produces severe memory impairment. This lesion, which in- to the amygdala, and the plus superscript (+) to the cortical cludes the hippocampal formation, amygdala, and adjacent tissue adjacent to each structure.) This lesion appears to con- cortex, including the parahippocampal gyrus (the H+A+ le- stitute an animal model of medial temporal lobe amnesia like sion), appears to constitute an animal model of human me- that exhibited by the well-studied patient H.M. (Scoville and dial temporal lobe amnesia. Reexamination of histological Milner, 1957). material from previously studied monkeys with H+A+ lesions The H+A+ lesion produces greater memory impairment than indicated that the perirhinal cortex had also sustained sig- a lesion limited to the hippocampal formation and parahip- nificant damage. Furthermore, recent neuroanatomical stud- pocampal cortex-the H+ lesion (Mishkin, 1978; Mahut et al., ies show that the perirhinal cortex and the closely associated 1982; Zola-Morgan and Squire, 1985, 1986; Zola-Morgan et al., parahippocampal cortex provide the major source of cortical 1989a). -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

The Pre/Parasubiculum: a Hippocampal Hub for Scene- Based Cognition? Marshall a Dalton and Eleanor a Maguire
Available online at www.sciencedirect.com ScienceDirect The pre/parasubiculum: a hippocampal hub for scene- based cognition? Marshall A Dalton and Eleanor A Maguire Internal representations of the world in the form of spatially which posits that one function of the hippocampus is to coherent scenes have been linked with cognitive functions construct internal representations of scenes in the ser- including episodic memory, navigation and imagining the vice of memory, navigation, imagination, decision-mak- future. In human neuroimaging studies, a specific hippocampal ing and a host of other functions [11 ]. Recent inves- subregion, the pre/parasubiculum, is consistently engaged tigations have further refined our understanding of during scene-based cognition. Here we review recent evidence hippocampal involvement in scene-based cognition. to consider why this might be the case. We note that the pre/ Specifically, a portion of the anterior medial hippocam- parasubiculum is a primary target of the parieto-medial pus is consistently engaged by tasks involving scenes temporal processing pathway, it receives integrated [11 ], although it is not yet clear why a specific subre- information from foveal and peripheral visual inputs and it is gion of the hippocampus would be preferentially contiguous with the retrosplenial cortex. We discuss why these recruited in this manner. factors might indicate that the pre/parasubiculum has privileged access to holistic representations of the environment Here we review the extant evidence, drawing largely from and could be neuroanatomically determined to preferentially advances in the understanding of visuospatial processing process scenes. pathways. We propose that the anterior medial portion of the hippocampus represents an important hub of an Address extended network that underlies scene-related cognition, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, and we generate specific hypotheses concerning the University College London, 12 Queen Square, London WC1N 3BG, UK functional contributions of hippocampal subfields. -

Involvement of Human Left Dorsolateral Prefrontal Cortex in Perceptual Decision Making Is Independent of Response Modality
Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality H. R. Heekeren*†‡§, S. Marrett¶, D. A. Ruff*, P. A. Bandettini*¶, and L. G. Ungerleider*ʈ *Laboratory of Brain and Cognition, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892-9663; †Max Planck Institute for Human Development, 14195 Berlin, Germany; ‡Max Planck Institute for Human Cognitive and Brain Sciences, 04103 Leipzig, Germany; §Berlin NeuroImaging Center, Charite´University Medicine Berlin, 10117 Berlin, Germany; and ¶Functional MRI Facility, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892-9663 Contributed by L. G. Ungerleider, May 12, 2006 Perceptual decision making typically entails the processing of such as the DLPFC, during high-motion-coherence trials, i.e., trials sensory signals, the formation of a decision, and the planning and in which the sensory evidence is greatest, than during low- execution of a motor response. Although recent studies in mon- coherence trials. keys and humans have revealed possible neural mechanisms for As in the visual system, in the somatosensory system, in a task perceptual decision making, much less is known about how the in which the monkey must decide which of two vibratory stimuli decision is subsequently transformed into a motor action and has a higher frequency, the monkey’s decision can be predicted whether or not the decision is represented at an abstract level, i.e., by subtracting the activities of two populations of sensory independently of the specific motor response. To address this neurons in the secondary somatosensory cortex (SII) that prefer issue, we used functional MRI to monitor changes in brain activity high and low frequencies, respectively (10). -

The Surgical Prognostic Significance of the Electroencephalographic Prediction of Ammon's Horn Sclerosis in Epileptics by W
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.21.1.24 on 1 February 1958. Downloaded from J. Neurol. Neurosurg. Psychiat., 1958, 21, 24. THE SURGICAL PROGNOSTIC SIGNIFICANCE OF THE ELECTROENCEPHALOGRAPHIC PREDICTION OF AMMON'S HORN SCLEROSIS IN EPILEPTICS BY W. A. KENNEDY and DENIS HILL BASED ON PATHOLOGICAL MATERIAL SUPPLIED BY J. B. CAVANAGH and A. MEYER From the Institute ofPsychiatry and the Guy's-Maudsley Neurosurgical Unit, London In a previous communication (Falconer, Hill, Jasper (1954) and recently by Rasmussen (1957). Meyer, Mitchell, and Pond, 1955) the results of The diagnostic and pathological data and the treating intractable temporal lobe epilepsy by tem- therapeutic effects of temporal lobectomy carried poral lobectomy were given. A survey was reported out on a larger series of 50 cases are now available of the clinical, electroencephalographic, radiological, and a preliminary report has been given (Falconer, and pathological findings in 31 cases. The follow-up Meyer, Hill, and Wilson, 1957). Following Earle etguest. Protected by copyright. study of the patients showed beneficial effects upon al. (1953) the pathological findings have been classi- the epilepsy and upon the personality disorder in a fied in two main groups. While all cases in which a proportion which compared very favourably with space-occupying lesion could be anticipated on most published series. The opinion was expressed clinical, radiological, or electroencephalographic that this was due to inclusion of the uncus, Ammon's grounds were excluded from the series, there were, horn, and possibly the amygdaloid nucleus in the nevertheless, 14 cases in which very small focal resected tissue. -

Subtemporal Transparahippocampal Amygdalohippocampectomy for Surgical Treatment of Mesial Temporal Lobe Epilepsy Technical Note
Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy Technical note T. S. Park, M.D., Blaise F. D. Bourgeois, M.D., Daniel L. Silbergeld, M.D., and W. Edwin Dodson, M.D. Department of Neurology and Neurological Surgery, Washington University School of Medicine, and St. Louis Children's Hospital, St. Louis, Missouri Amygdalohippocampectomy (AH) is an accepted surgical option for treatment of medically refractory mesial temporal lobe epilepsy. Operative approaches to the amygdala and hippocampus that previously have been reported include: the sylvian fissure, the superior temporal sulcus, the middle temporal gyrus, and the fusiform gyrus. Regardless of the approach, AH permits not only extirpation of an epileptogenic focus in the amygdala and anterior hippocampus, but interruption of pathways of seizure spread via the entorhinal cortex and the parahippocampal gyrus. The authors report a modification of a surgical technique for AH via the parahippocampal gyrus, in which excision is limited to the anterior hippocampus, amygdala and parahippocampal gyrus while preserving the fusiform gyrus and the rest of the temporal lobe. Because transparahippocampal AH avoids injury to the fusiform gyrus and the lateral temporal lobe, it can be performed without intracarotid sodium amobarbital testing of language dominance and language mapping. Thus the operation would be particularly suitable for pediatric patients in whom intraoperative language mapping before resection is difficult. Key Words * amygdalohippocampectomy * complex partial seizure * parahippocampal gyrus * subtemporal approach Currently several different variations of temporal lobe resections are used for medically intractable complex partial seizures.[4,6,8,18,21,30,34] Among these operations is amygdalohippocampectomy (AH), first described in 1958 by Niemeyer,[16] who approached the amygdala and hippocampus through an incision on the middle temporal gyrus. -

Default Mode Network in Childhood Autism Posteromedial Cortex Heterogeneity and Relationship with Social Deficits
ARCHIVAL REPORT Default Mode Network in Childhood Autism: Posteromedial Cortex Heterogeneity and Relationship with Social Deficits Charles J. Lynch, Lucina Q. Uddin, Kaustubh Supekar, Amirah Khouzam, Jennifer Phillips, and Vinod Menon Background: The default mode network (DMN), a brain system anchored in the posteromedial cortex, has been identified as underconnected in adults with autism spectrum disorder (ASD). However, to date there have been no attempts to characterize this network and its involvement in mediating social deficits in children with ASD. Furthermore, the functionally heterogeneous profile of the posteromedial cortex raises questions regarding how altered connectivity manifests in specific functional modules within this brain region in children with ASD. Methods: Resting-state functional magnetic resonance imaging and an anatomically informed approach were used to investigate the functional connectivity of the DMN in 20 children with ASD and 19 age-, gender-, and IQ-matched typically developing (TD) children. Multivariate regression analyses were used to test whether altered patterns of connectivity are predictive of social impairment severity. Results: Compared with TD children, children with ASD demonstrated hyperconnectivity of the posterior cingulate and retrosplenial cortices with predominately medial and anterolateral temporal cortex. In contrast, the precuneus in ASD children demonstrated hypoconnectivity with visual cortex, basal ganglia, and locally within the posteromedial cortex. Aberrant posterior cingulate cortex hyperconnectivity was linked with severity of social impairments in ASD, whereas precuneus hypoconnectivity was unrelated to social deficits. Consistent with previous work in healthy adults, a functionally heterogeneous profile of connectivity within the posteromedial cortex in both TD and ASD children was observed. Conclusions: This work links hyperconnectivity of DMN-related circuits to the core social deficits in young children with ASD and highlights fundamental aspects of posteromedial cortex heterogeneity. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

An Event-Related Functional Magnetic Resonance Imaging Study
Neural Correlates of Memory for Items and for Associations: An Event-related Functional Magnetic Resonance Imaging Study Ame´lie M. Achim and Martin Lepage Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/17/4/652/1757197/0898929053467578.pdf by guest on 18 May 2021 Abstract & Although results from cognitive psychology, neuropsychol- coding, greater prefrontal, hippocampal, and parietal activation ogy, and behavioral neuroscience clearly suggest that item was observed for associations, but no significant activation was and associative information in memory rely on partly different observed for items at the selected threshold. During recog- brain regions, little is known concerning the differences and nition, greater activation was observed for associative trials in similarities that exist between these two types of information as the left dorsolateral prefrontal cortex and superior parietal a function of memory stage (i.e., encoding and retrieval). We lobules bilaterally, whereas item recognition trials showed used event-related functional magnetic resonance imaging to greater activation of bilateral frontal regions, bilateral anterior assess neural correlates of item and associative encoding and medial temporal areas, and the right temporo-parietal junction. retrieval of simple images in 18 healthy subjects. During en- Post hoc analyses suggested that the anterior medial temporal coding, subjects memorized items and pairs. During retrieval, activation observed during item recognition was driven mainly subjects made item recognition judgments (old vs. new) and by new items, confirming a role for this structure in novelty de- associative recognition judgments (intact vs. rearranged). Rel- tection. These results suggest that although some structures ative to baseline, item and associative trials activated bilateral such as the medial temporal and prefrontal cortex play a gen- medial temporal and prefrontal regions during both encoding eral role in memory, the pattern of activation in these regions and retrieval. -

Functional Connectivity of the Precuneus in Unmedicated Patients with Depression
Biological Psychiatry: CNNI Archival Report Functional Connectivity of the Precuneus in Unmedicated Patients With Depression Wei Cheng, Edmund T. Rolls, Jiang Qiu, Deyu Yang, Hongtao Ruan, Dongtao Wei, Libo Zhao, Jie Meng, Peng Xie, and Jianfeng Feng ABSTRACT BACKGROUND: The precuneus has connectivity with brain systems implicated in depression. METHODS: We performed the first fully voxel-level resting-state functional connectivity (FC) neuroimaging analysis of depression of the precuneus, with 282 patients with major depressive disorder and 254 control subjects. RESULTS: In 125 unmedicated patients, voxels in the precuneus had significantly increased FC with the lateral orbitofrontal cortex, a region implicated in nonreward that is thereby implicated in depression. FC was also increased in depression between the precuneus and the dorsolateral prefrontal cortex, temporal cortex, and angular and supramarginal areas. In patients receiving medication, the FC between the lateral orbitofrontal cortex and precuneus was decreased back toward that in the control subjects. In the 254 control subjects, parcellation revealed superior anterior, superior posterior, and inferior subdivisions, with the inferior subdivision having high connectivity with the posterior cingulate cortex, parahippocampal gyrus, angular gyrus, and prefrontal cortex. It was the ventral subdivision of the precuneus that had increased connectivity in depression with the lateral orbitofrontal cortex and adjoining inferior frontal gyrus. CONCLUSIONS: The findings support the theory that the system in the lateral orbitofrontal cortex implicated in the response to nonreceipt of expected rewards has increased effects on areas in which the self is represented, such as the precuneus. This may result in low self-esteem in depression. The increased connectivity of the precuneus with the prefrontal cortex short-term memory system may contribute to the rumination about low self-esteem in depression. -
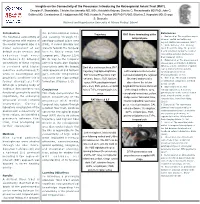
Insights on the Connectivity of the Precuneus: Introducing the Retrosplenial Aslant Tract (RAT)
Insights on the Connectivity of the Precuneus: Introducing the Retrosplenial Aslant Tract (RAT). Georgios P. Skandalakis; Christos Koutsarnakis MD, MSc; Aristotelis Kalyvas; Dimitris G. Placantonakis MD PhD; John G. Golfinos MD; Constantinos G. Hadjipanayis MD PhD; Kostas N. Fountas MD PhD FAANS; Eftychia Z. Kapsalaki MD; George S. Stranjalis National and Kapodistrian University of Athens Medical School Introduction the parieto-occipital sulcus, References Trajectory RAT fibers terminating at the The functional connectivity of and passing through the 1. Epstein et al. The cognitive map in temporal pole humans: spatial navigation and the precuneus with regions of parahippocampal place area beyond. Nature neuroscience. (2017) the medial temporal lobe is a (PPA), it curves laterally and 2. Wade-Bohleber et al. Thinking major component of our projects towards the temporal about the past to shape the present: default mode network and horn to finally reach the Neural activation during the recall of relationship episodes. Behavioural underlies high order temporal pole. (Figures 1,3,4) Brain Research (2018). functions.(1-5) Stronger On its way to the temporal 3. Hebscher et al. The precuneus and connectivity of these regions pole this tracts also displays hippocampus contribute to individual differences in the unfolding of spatial is corelated with higher connections with the lingual, Dark blue continuous lines, RAT representations during episodic cognitive performances(7) parahippocampal and fusiform trajectory; highlighted light blue, Left hemisphere infero-medial autobiographical memory. while in neurological and gyri, inferior longitudinal RAT Cortical Projections; CaF, view demonstrating the regional Neuropsychologia. (2018) psychiatric conditions this is fasciculus and hippocampal calcarine fissure; FuG, fusiform fiber tract anatomy after 4.