Invasive Neuromodulation As a Possible Treatment for Tinnitus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

'What' but Not 'Where' Auditory Processing Pathway
NeuroImage 82 (2013) 295–305 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Emotion modulates activity in the ‘what’ but not ‘where’ auditory processing pathway James H. Kryklywy d,e, Ewan A. Macpherson c,f, Steven G. Greening b,e, Derek G.V. Mitchell a,b,d,e,⁎ a Department of Psychiatry, University of Western Ontario, London, Ontario N6A 5A5, Canada b Department of Anatomy & Cell Biology, University of Western Ontario, London, Ontario N6A 5A5, Canada c National Centre for Audiology, University of Western Ontario, London, Ontario N6A 5A5, Canada d Graduate Program in Neuroscience, University of Western Ontario, London, Ontario N6A 5A5, Canada e Brain and Mind Institute, University of Western Ontario, London, Ontario N6A 5A5, Canada f School of Communication Sciences and Disorders, University of Western Ontario, London, Ontario N6A 5A5, Canada article info abstract Article history: Auditory cortices can be separated into dissociable processing pathways similar to those observed in the vi- Accepted 8 May 2013 sual domain. Emotional stimuli elicit enhanced neural activation within sensory cortices when compared to Available online 24 May 2013 neutral stimuli. This effect is particularly notable in the ventral visual stream. Little is known, however, about how emotion interacts with dorsal processing streams, and essentially nothing is known about the impact of Keywords: emotion on auditory stimulus localization. In the current study, we used fMRI in concert with individualized Auditory localization Emotion auditory virtual environments to investigate the effect of emotion during an auditory stimulus localization fi Auditory processing pathways task. Surprisingly, participants were signi cantly slower to localize emotional relative to neutral sounds. -

Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment
The Journal of Neuroscience, December 1989, 9(12): 4355-4370 Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment Stuart Zola-Morgan,’ Larry Ft. Squire,’ David G. Amaral,2 and Wendy A. Suzuki2J Veterans Administration Medical Center, San Diego, California, 92161, and Department of Psychiatry, University of California, San Diego, La Jolla, California 92093, The Salk Institute, San Diego, California 92136, and 3Group in Neurosciences, University of California, San Diego, La Jolla, California 92093 In monkeys, bilateral damage to the medial temporal region Moss, 1984). (In this notation, H refers to the hippocampus, A produces severe memory impairment. This lesion, which in- to the amygdala, and the plus superscript (+) to the cortical cludes the hippocampal formation, amygdala, and adjacent tissue adjacent to each structure.) This lesion appears to con- cortex, including the parahippocampal gyrus (the H+A+ le- stitute an animal model of medial temporal lobe amnesia like sion), appears to constitute an animal model of human me- that exhibited by the well-studied patient H.M. (Scoville and dial temporal lobe amnesia. Reexamination of histological Milner, 1957). material from previously studied monkeys with H+A+ lesions The H+A+ lesion produces greater memory impairment than indicated that the perirhinal cortex had also sustained sig- a lesion limited to the hippocampal formation and parahip- nificant damage. Furthermore, recent neuroanatomical stud- pocampal cortex-the H+ lesion (Mishkin, 1978; Mahut et al., ies show that the perirhinal cortex and the closely associated 1982; Zola-Morgan and Squire, 1985, 1986; Zola-Morgan et al., parahippocampal cortex provide the major source of cortical 1989a). -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -
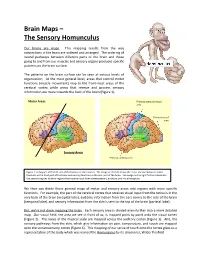
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Subdivisions of Auditory Cortex and Processing Streams in Primates
Colloquium Subdivisions of auditory cortex and processing streams in primates Jon H. Kaas*† and Troy A. Hackett‡ Departments of †Psychology and ‡Hearing and Speech Sciences, Vanderbilt University, Nashville, TN 37240 The auditory system of monkeys includes a large number of histochemical studies in chimpanzees and humans, and nonin- interconnected subcortical nuclei and cortical areas. At subcortical vasive functional studies in humans. levels, the structural components of the auditory system of mon- keys resemble those of nonprimates, but the organization at The Core Areas of Auditory Cortex cortical levels is different. In monkeys, the ventral nucleus of the Originally, auditory cortex of monkeys was thought to be orga- medial geniculate complex projects in parallel to a core of three nized much as in cats, with a single primary area, AI, in the primary-like auditory areas, AI, R, and RT, constituting the first cortex of the lower bank of the lateral sulcus and a second area, stage of cortical processing. These areas interconnect and project AII, deeper in the sulcus (e.g., ref. 3). This concept fits well with to the homotopic and other locations in the opposite cerebral the early view that auditory, somatosensory, and visual systems hemisphere and to a surrounding array of eight proposed belt all have two fields. However, we now know that primates have areas as a second stage of cortical processing. The belt areas in turn a number of sensory representations for each modality, and project in overlapping patterns to a lateral parabelt region with at several somatosensory and auditory fields can be considered least rostral and caudal subdivisions as a third stage of cortical primary or primary like in character. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

Involvement of Human Left Dorsolateral Prefrontal Cortex in Perceptual Decision Making Is Independent of Response Modality
Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality H. R. Heekeren*†‡§, S. Marrett¶, D. A. Ruff*, P. A. Bandettini*¶, and L. G. Ungerleider*ʈ *Laboratory of Brain and Cognition, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892-9663; †Max Planck Institute for Human Development, 14195 Berlin, Germany; ‡Max Planck Institute for Human Cognitive and Brain Sciences, 04103 Leipzig, Germany; §Berlin NeuroImaging Center, Charite´University Medicine Berlin, 10117 Berlin, Germany; and ¶Functional MRI Facility, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892-9663 Contributed by L. G. Ungerleider, May 12, 2006 Perceptual decision making typically entails the processing of such as the DLPFC, during high-motion-coherence trials, i.e., trials sensory signals, the formation of a decision, and the planning and in which the sensory evidence is greatest, than during low- execution of a motor response. Although recent studies in mon- coherence trials. keys and humans have revealed possible neural mechanisms for As in the visual system, in the somatosensory system, in a task perceptual decision making, much less is known about how the in which the monkey must decide which of two vibratory stimuli decision is subsequently transformed into a motor action and has a higher frequency, the monkey’s decision can be predicted whether or not the decision is represented at an abstract level, i.e., by subtracting the activities of two populations of sensory independently of the specific motor response. To address this neurons in the secondary somatosensory cortex (SII) that prefer issue, we used functional MRI to monitor changes in brain activity high and low frequencies, respectively (10). -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -
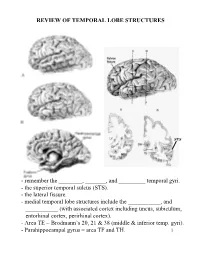
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

Default Mode Network in Childhood Autism Posteromedial Cortex Heterogeneity and Relationship with Social Deficits
ARCHIVAL REPORT Default Mode Network in Childhood Autism: Posteromedial Cortex Heterogeneity and Relationship with Social Deficits Charles J. Lynch, Lucina Q. Uddin, Kaustubh Supekar, Amirah Khouzam, Jennifer Phillips, and Vinod Menon Background: The default mode network (DMN), a brain system anchored in the posteromedial cortex, has been identified as underconnected in adults with autism spectrum disorder (ASD). However, to date there have been no attempts to characterize this network and its involvement in mediating social deficits in children with ASD. Furthermore, the functionally heterogeneous profile of the posteromedial cortex raises questions regarding how altered connectivity manifests in specific functional modules within this brain region in children with ASD. Methods: Resting-state functional magnetic resonance imaging and an anatomically informed approach were used to investigate the functional connectivity of the DMN in 20 children with ASD and 19 age-, gender-, and IQ-matched typically developing (TD) children. Multivariate regression analyses were used to test whether altered patterns of connectivity are predictive of social impairment severity. Results: Compared with TD children, children with ASD demonstrated hyperconnectivity of the posterior cingulate and retrosplenial cortices with predominately medial and anterolateral temporal cortex. In contrast, the precuneus in ASD children demonstrated hypoconnectivity with visual cortex, basal ganglia, and locally within the posteromedial cortex. Aberrant posterior cingulate cortex hyperconnectivity was linked with severity of social impairments in ASD, whereas precuneus hypoconnectivity was unrelated to social deficits. Consistent with previous work in healthy adults, a functionally heterogeneous profile of connectivity within the posteromedial cortex in both TD and ASD children was observed. Conclusions: This work links hyperconnectivity of DMN-related circuits to the core social deficits in young children with ASD and highlights fundamental aspects of posteromedial cortex heterogeneity.