WHO Immunological Basis for Immunization Series
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CLINICAL TRIALS Safety and Immunogenicity of a Nicotine Conjugate Vaccine in Current Smokers
CLINICAL TRIALS Safety and immunogenicity of a nicotine conjugate vaccine in current smokers Immunotherapy is a novel potential treatment for nicotine addiction. The aim of this study was to assess the safety and immunogenicity of a nicotine conjugate vaccine, NicVAX, and its effects on smoking behavior. were recruited for a noncessation treatment study and assigned to 1 of 3 doses of the (68 ؍ Smokers (N nicotine vaccine (50, 100, or 200 g) or placebo. They were injected on days 0, 28, 56, and 182 and monitored for a period of 38 weeks. Results showed that the nicotine vaccine was safe and well tolerated. Vaccine immunogenicity was dose-related (P < .001), with the highest dose eliciting antibody concentrations within the anticipated range of efficacy. There was no evidence of compensatory smoking or precipitation of nicotine withdrawal with the nicotine vaccine. The 30-day abstinence rate was significantly different across with the highest rate of abstinence occurring with 200 g. The nicotine vaccine appears ,(02. ؍ the 4 doses (P to be a promising medication for tobacco dependence. (Clin Pharmacol Ther 2005;78:456-67.) Dorothy K. Hatsukami, PhD, Stephen Rennard, MD, Douglas Jorenby, PhD, Michael Fiore, MD, MPH, Joseph Koopmeiners, Arjen de Vos, MD, PhD, Gary Horwith, MD, and Paul R. Pentel, MD Minneapolis, Minn, Omaha, Neb, Madison, Wis, and Rockville, Md Surveys show that, although about 41% of smokers apy, is about 25% on average.2 Moreover, these per- make a quit attempt each year, less than 5% of smokers centages most likely exaggerate the efficacy of are successful at remaining abstinent for 3 months to a intervention because these trials are typically composed year.1 Smokers seeking available behavioral and phar- of subjects who are highly motivated to quit and who macologic therapies can enhance successful quit rates are free of complicating diagnoses such as depression 2 by 2- to 3-fold over control conditions. -

Program Evaluation Key Outcomes and Addressing Remaining
Vaccine 31S (2013) J73–J78 Contents lists available at ScienceDirect Vaccine jou rnal homepage: www.elsevier.com/locate/vaccine Key outcomes and addressing remaining challenges—Perspectives from a final ଝ evaluation of the China GAVI project a,1 a,1 a,1 a,1 b c Weizhong Yang , Xiaofeng Liang , Fuqiang Cui , Li Li , Stephen C. Hadler , Yvan J. Hutin , d a,∗ Mark Kane , Yu Wang a Chinese Center for Disease Control and Prevention, Beijing, China b Centers for Disease Control and Prevention, Atlanta, USA c Europe Center for Disease Control and Prevention, Stockholm, Sweden d Mercer Island, Washington, USA a r t a b i c s t l r e i n f o a c t Article history: During the China GAVI project, implemented between 2002 and 2010, more than 25 million children Received 6 June 2012 received hepatitis B vaccine with the support of project, and the vaccine proved to be safe and effective. Received in revised form 24 July 2012 With careful consideration for project savings, China and GAVI continually adjusted the budget, addi- Accepted 24 September 2012 tionally allowing the project to spend operational funds to support demonstration projects to improve timely birth dose (TBD), conduct training of EPI staff, and to monitor the project impact. Results from the final evaluation indicated the achievement of key outcomes. As a result of government co-investment, Keywords: human resources at county level engaged in hepatitis B vaccination increased from 29 per county on GAVI Project average in 2002 to 66 in 2009. All project counties funded by the GAVI project use auto-disable syringes Outcomes for hepatitis B vaccination and other vaccines. -

Summary Guide to Tetanus Prophylaxis in Routine Wound Management
Summary Guide to Tetanus Prophylaxis in Routine Wound Management ASSESS WOUND A clean, minor wound All other wounds (contaminated with dirt, feces, saliva, soil; puncture wounds; avulsions; wounds resulting from flying or crushing objects, animal bites, burns, frostbite) Has patient completed a primary Has patient completed a primary tetanus diphtheria series?1, 7 tetanus diphtheria series?1, 7 No/Unknown Yes No/Unknown Yes Administer vaccine today.2,3,4 Was the most recent Administer vaccine and Was the most Instruct patient to complete dose within the past tetanus immune gobulin recent dose within series per age-appropriate 10 years? (TIG) now.2,4,5,6,7 the past 5 years?7 vaccine schedule. No Yes No Yes Vaccine not needed today. Vaccine not needed today. Administer vaccine today.2,4 2,4 Patient should receive next Administer vaccine today. Patient should receive next Patient should receive next dose Patient should receive next dose dose at 10-year interval after dose at 10-year interval after per age-appropriate schedule. per age-appropriate schedule. last dose. last dose. 1 A primary series consists of a minimum of 3 doses of tetanus- and diphtheria- 4 Tdap* is preferred for persons 11 through 64 years of age if using Adacel* or 10 years of containing vaccine (DTaP/DTP/Tdap/DT/Td). age and older if using Boostrix* who have never received Tdap. Td is preferred to tetanus 2 Age-appropriate vaccine: toxoid (TT) for persons 7 through 9 years, 65 years and older, or who have received a • DTaP for infants and children 6 weeks up to 7 years of age (or DT pediatric if Tdap previously. -

Vaccines 101 the Very Last Day That I Was a Pediatric Resident. Um, Many
Vaccines 101 The very last day that I was a pediatric resident. Um, many years ago, a toddler walked into the emergency room and uh, and progressively got sicker and sicker. That's Dr. Katherine Edwards, a world expert in pediatric infectious disease in vaccinology. She's also a professor of pediatrics at Vanderbilt university and she's been working on vaccines for 40 years. I did a spinal tap on her and realized that she had Haemophilus influenza, typ e B meningitis, Haemophilus influenza, type B or HIB is it bacteria normally found in our nose and throat that can lead to very serious life threatening infections and no matter what I did in that day and into the night in terms of prompt antibiotics and she'd just been sick a few hours and, and fluids and all the, you know, ventilators and all the best things that modern medicine, she died, the vaccine for hip was not available until the 1990s. And until it did become available, hip disease affected approximately 25,000 children each year with things like meningitis, pneumonia, and bloodstream infections. And at that time in the hospital that I was practicing at any one time, there were generally five or six patients that had Haemophilus meningitis or invasive disease or some complication of this particular infection. And we knew that from the basic science that if you had antibody to the capsule or to the coat of the organism, that you were protected from disease. But we really didn't know how to make little kids make antibody. -

Commonly Administered Vaccines
Commonly Administered Vaccines CPT CVX Vaccine Type Brand Name Manufacturer PhilaVax Display Name Description Code Code Combination Vaccines Diptheria, tetanus toxoids and acellular pertussis vaccine, Hepati- DTaP-HepB-IPV Pediarix® GlaxoSmithKline 90723 110 DTaP-HepB-IPV tis B and poliovirus vaccine, inactivated Diptheria, tetanus toxoids and acellular pertussis vaccine and DTaP-Hib TriHIBit® Sanofi Pasteur 90721 50 DTaP-Hib (TriHIBit) Haemophilus influenzae type b conjugate vaccine Diptheria, tetanus toxoids and acellular pertussis vaccine, Hae- DTaP-Hib-IPV Pentacel® Sanofi Pasteur 90698 120 DTaP-Hib-IPV mophilus influenzae type b, and poliovirus vaccine, inactivated Diptheria, tetanus toxoids and acellular pertussis vaccine, and DTaP-IPV Kinrix® GlaxoSmithKline 90696 130 DTaP-IPV (KINRIX) poliovirus vaccine, inactivated HepA-HepB TWINRIX® GlaxoSmithKline 90636 104 HepA/B (TWINRIX) Hepatisis A and Hepatitis B vaccine, adult dosage Hepatitis B and Hemophilus influenza b vaccine, for intramuscular HepB-Hib Comvax® Merck 90748 51 Hib-Hep B (COMVAX) use Haemophilus influenza b and meningococcal sero groups C and Y MeningC/Y-Hib Menhibrix® GlaxoSmithKline 90644 148 Meningococcal-Hib vaccine, 4 dose series Diphtheria, Tetanus and Pertussis DTaP Infanrix® GlaxoSmithKline 90700 20 DTaP (INFANRIX) Diptheria, tetanus toxoids and acellular pertussis vaccine DTaP, 5 Pertussis Antigens Daptacel® Sanofi Pasteur 90700 106 DTaP (DAPTACEL) Diptheria, tetanus toxoids and acellular pertussis vaccine Tetanus toxoid, reduced diphtheria toxoid, and acellular -

Vaccine Hesitancy
Vaccine Hesitancy Dr Brenda Corcoran National Immunisation Office Presentation Outline An understanding of the following principles: • Overview of immunity • Different types of vaccines and vaccine contents • Vaccine failures • Time intervals between vaccine doses • Vaccine overload • Adverse reactions • Herd immunity Immunity Immunity • The ability of the human body to protect itself from infectious disease The immune system • Cells with a protective function in the – bone marrow – thymus – lymphatic system of ducts and nodes – spleen –blood Types of immunity Source: http://en.wikipedia.org/wiki/Immunological_memory Natural (innate) immunity Non-specific mechanisms – Physical barriers • skin and mucous membranes – Chemical barriers • gastric and digestive enzymes – Cellular and protein secretions • phagocytes, macrophages, complement system ** No “memory” of protection exists afterwards ** Passive immunity – adaptive mechanisms Natural • maternal transfer of antibodies to infant via placenta Artificial • administration of pre- formed substance to provide immediate but short-term protection (antitoxin, antibodies) Protection is temporary and wanes with time (usually few months) Active immunity – adaptive mechanisms Natural • following contact with organism Artificial • administration of agent to stimulate immune response (immunisation) Acquired through contact with an micro-organism Protection produced by individual’s own immune system Protection often life-long but may need boosting How vaccines work • Induce active immunity – Immunity and immunologic memory similar to natural infection but without risk of disease • Immunological memory allows – Rapid recognition and response to pathogen – Prevent or modify effect of disease Live attenuated vaccines Weakened viruses /bacteria – Achieved by growing numerous generations in laboratory – Produces long lasting immune response after one or two doses – Stimulates immune system to react as it does to natural infection – Can cause mild form of the disease (e.g. -

Immunization Policies and Procedures Manual
Immunization Policies and Procedures Manual Louisiana Department of Health Office of Public Health Immunization Program Revised September 2017 i Center for Community and Preventive Health Bureau of Infectious Diseases Immunization Program TABLE OF CONTENTS I. POLICY AND GENERAL CLINIC POLICY ............................................................................................................................. 1 PURPOSE ........................................................................................................................................................................................... 1 POLICY ON CLINIC SCHEDULING ............................................................................................................................................ 2 POLICY ON PUBLICITY FOR IMMUNIZATION ACTIVITIES .............................................................................................. 4 POLICY ON EDUCATIONAL ACTIVITIES (HEALTH EDUCATION IN IMMUNIZATION CLINICS) .......................... 5 POLICY ON CHECKING IMMUNIZATION STATUS OF ALL CHILDREN RECEIVING SERVICES THROUGH THE HEALTH DEPARTMENT ...................................................................................................................................................... 6 POLICY ON MAXIMIZING TIME SPENT WITH PARENTS DURING IMMUNIZATION CLINICS ............................... 7 POLICY ON ASSISTANCE TO FOREIGN TRAVELERS .......................................................................................................... 9 II. POLICY -

The Challenge of Achieving Universal Access to Vaccines
Keynote Address: The Challenge of Achieving Universal Access to Vaccines WIPO & AMF Seth Berkley M.D, CEO 8 November 2017 www.gavi.org 1 Vaccine landscape WIPO 8 November 2017 The world before vaccines Examples of major disease outbreaks Polio Cholera New York pandemic 1916 Europe 6,000 deaths 1829-1851 >200,000 deaths Yellow fever Philadelphia Smallpox 1793 epidemic >5,000 India deaths 1974 15,000 deaths Flu pandemic 1918-1920 > 50-100 million deaths worldwide WIPO 8 November 2017 History of vaccine development Source: Delaney et.al. 2014 WIPO 8 November 2017 Cumulative number of vaccines developed licensed 50+ vaccines Lyme OspA, protein (1998) Tuberculosis (Bacille Calmette-Guérin) Pneumococcal conjugate, heptavalent* (2000) 1927 Typhus Varicella (1995) Meningococcal quadrivalent conjugates* (2005) Japanese encephalitis (Vera-cell) (2009) 1938 Cholera (recombinant Toxin B) # (1993) Cholera (WC only) (2009) Tetanus toxoid Cholera (WC-rBC) (1991) Human papillomavirus recombinant bivalent (2009) Influenza H. influenzaeconjugate* (1987) Quadrivalent influenza vaccines (2012) Typhoid Cholera Pertussis 1936 Meningococcal C and Y and 1886 1896 1926 H. influenza type B polysaccharide (1985) Haemophilusinfluenza type b (2012) Adenovirus (1980) Meningococcal B (2013) Smallpox Diphtheria Yellow Rabies, cell culture (1980) Influenza vaccine (baculovirus) (2013) 1798 toxoid Fever Typhoid conjugate vaccine (2013) Rabies Plague Meningococcal polysaccharides (1974) 1923 1935 Human papillomavirus, 9-valent (2014) 1885 Rubella, live (1969) 1897 vaccines -

UNHCR COVID-19 Global Emergency Response
GLOBAL COVID-19 EMERGENCY RESPONSE 17 February 2021 UNHCR COVID-19 Preparedness and Response Highlights COVID-19 update ■ UNHCR and Gavi, the Vaccine Alliance, signed a Memorandum of Over 46,000 Understanding (MoU) on 03 February 2020, with the overall goal of ensuring reported cases refugees and other forcibly displaced can access vaccines on par with of COVID-19 nationals. The MoU also looks at expanding coverage and quality of among forcibly displaced people immunization services, promoting equity in access and uptake of vaccines, and strengthening health systems at community and primary care level. across 103 countries ■ Jordan has become one of the world’s first countries to start COVID-19 vaccinations for refugees, including vaccinations in Za’atari refugee camp on 15 February. UNHCR has been working with the Jordanian government to increase of vaccinate refugees and provide critical health, sanitation, hygiene and logistical some 7,500 cases compared support. UNHCR appeals to all countries to follow suit and include refugees in to the previous their national vaccination drives in line with COVAX allocation principles. reporting period ■ In 2020, 39.4 million persons of concern received COVID-19 assistance (numbers as of 08 February including access to protection services, shelter, health, and education. This 2021) includes over 8.5 million individuals who received cash assistance. ■ Despite an estimated 1.44 million refugees in urgent need of resettlement globally, less than 23,000 were resettled through UNHCR last year. These are the lowest refugee resettlement numbers UNHCR has witnessed in almost two decades. The drop stems from low quotas put forward by states, as well as the impact of COVID-19, which delayed departures and programmes. -

UNICEF Immunization Roadmap 2018-2030
UNICEF IMMUNIZATION ROADMAP 2018–2030 Cover: ©UNICEF/UN065768/Khouder Al-Issa A health worker vaccinates 3-year-old Rahaf in Tareek Albab neighborhood in the eastern part of Aleppo city. UNICEF IMMUNIZATION ROADMAP 2018–2030 Photograph credits: Pages vi-vii: © Shutterstock/thi Page 5: © UNICEF/UN060913/Al-Issa Page 6: © UNICEF/UNI41364/Estey Page 10: © UNICEF/UN061432/Dejongh Page 14: © UNICEF/UNI76541/Holmes Page 18: © UNICEF/UN0125829/Sharma Page 24: © UNICEF/UN059884/Zar Mon Page 26: © UNICEF/UN065770/Al-Issa Page 33: © UNICEF/UN0143438/Alhariri Page 34: © UNICEF/UNI45693/Estey Page 38: © UNICEF/UNI77040/Holmes Page 40: © UNICEF/UN0125861/Sharma Page 44: © UNICEF/UNI41211/Holmes © United Nations Children’s Fund (UNICEF), September 2018 Permission is required to reproduce any part of this publication. Permission will be freely granted to educational or non-profit organizations. Please contact: Health Section, Immunization Team UNICEF 3 United Nations Plaza New York, NY 100017 Contents Abbreviations viii Executive summary 1 1. Introduction 7 1.1 Background 7 1.2 Developing the Roadmap 9 2. Key considerations informing the UNICEF Immunization Roadmap 11 2.1 Key drivers of immunization through 2030 11 2.2 Evolving immunization partnerships 15 2.3 UNICEF’s comparative advantage in immunization 16 3. What is new in this Roadmap? 19 3.1 Immunization coverage as a tracer indicator of child equity 19 3.2 Key areas of work 21 4. Roadmap programming framework 27 4.1 Vision and impact statements 27 4.2 Programming principles 27 4.3 Context-driven responses 27 4.4 Objectives and priorities 29 4.5 Populations and platforms 32 4.6 Country-level immunization strategies 32 5. -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -
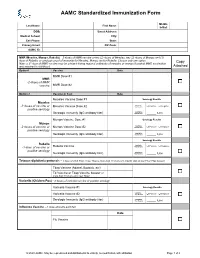
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC.