Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children
March 19, 1999 / Vol. 48 / No. RR-2 Recommendations and Reports Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children Recommendations of the Advisory Committee on Immunization Practices (ACIP) Continuing Education Examination Education Continuing Inside: U.S. DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Disease Control and Prevention (CDC) Atlanta, Georgia 30333 The MMWR series of publications is published by the Epidemiology Program Office, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30333. SUGGESTED CITATION Centers for Disease Control and Prevention. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1999;48(No. RR-2):[inclu- sive page numbers]. Centers for Disease Control and Prevention....................Jeffrey P. Koplan, M.D., M.P.H. Director The material in this report was prepared for publication by National Center for Infectious Diseases.................................. James M. Hughes, M.D. Director Division of Viral and Rickettsial Diseases ................. Brian W. J. Mahy, Ph.D., Sc.D. Director National Immunization Program ...........................................Walter A. Orenstein, M.D. Director Epidemiology and Surveillance Division ...........................John R. Livengood, M.D. Director The production of this report as an MMWR serial publication was coordinated in Epidemiology Program Office.................................... Stephen B. Thacker, M.D., M.Sc. Director Office of Scientific and Health Communications ......................John W. Ward, M.D. Director Editor, MMWR Series Recommendations and Reports................................... Suzanne M. Hewitt, M.P.A. Managing Editor Valerie R. Johnson Project Editor Morie M. Higgins Visual Information Specialist Vol. 48 / No. RR-2 MMWR i Contents Clinical and Epidemiologic Features of Rotavirus Disease............................................. -

Food Handler Exclusion Guidelines
Food Handler Exclusion Guidelines A guide for determining suitable exclusion periods for ill food handlers November 2017 Food handler exclusion guidelines Published by the State of Queensland (Queensland Health), November 2017 This document is licensed under a Creative Commons Attribution 3.0 Australia licence. To view a copy of this licence, visit creativecommons.org/licenses/by/3.0/au © State of Queensland (Queensland Health) 2017 You are free to copy, communicate and adapt the work, as long as you attribute the State of Queensland (Queensland Health). For more information contact: Food Safety Standards and Regulation, Department of Health, GPO Box 48, Brisbane QLD 4001, email [email protected], phone 07 3328 9310. An electronic version of this document is available at www.health.qld.gov.au. Disclaimer: The content presented in this publication is distributed by the Queensland Government as an information source only. The State of Queensland makes no statements, representations or warranties about the accuracy, completeness or reliability of any information contained in this publication. The State of Queensland disclaims all responsibility and all liability (including without limitation for liability in negligence) for all expenses, losses, damages and costs you might incur as a result of the information being inaccurate or incomplete in any way, and for any reason reliance was placed on such information. Food Handler Exclusion Guidelines - ii - Contents 1. Introduction ............................................................................................... -

PEDIARIX Is a Vaccine
HIGHLIGHTS OF PRESCRIBING INFORMATION • If Guillain-Barré syndrome occurs within 6 weeks of receipt of a prior These highlights do not include all the information needed to use vaccine containing tetanus toxoid, the decision to give PEDIARIX should PEDIARIX safely and effectively. See full prescribing information for be based on potential benefits and risks. (5.2) PEDIARIX. • The tip caps of the prefilled syringes contain natural rubber latex which may cause allergic reactions. (5.3) PEDIARIX [Diphtheria and Tetanus Toxoids and Acellular Pertussis • Syncope (fainting) can occur in association with administration of Adsorbed, Hepatitis B (Recombinant) and Inactivated Poliovirus injectable vaccines, including PEDIARIX. Procedures should be in place Vaccine], Suspension for Intramuscular Injection to avoid falling injury and to restore cerebral perfusion following Initial U.S. Approval: 2002 syncope. (5.4) • If temperature ≥105°F, collapse or shock-like state, or persistent, ----------------------------- INDICATIONS AND USAGE ---------------------------- PEDIARIX is a vaccine indicated for active immunization against diphtheria, inconsolable crying lasting ≥3 hours have occurred within 48 hours after tetanus, pertussis, infection caused by all known subtypes of hepatitis B virus, receipt of a pertussis-containing vaccine, or if seizures have occurred and poliomyelitis. PEDIARIX is approved for use as a 3-dose series in infants within 3 days after receipt of a pertussis-containing vaccine, the decision born of hepatitis B surface antigen (HBsAg)-negative mothers. PEDIARIX to give PEDIARIX should be based on potential benefits and risks. (5.5) may be given as early as 6 weeks of age through 6 years of age (prior to the • For children at higher risk for seizures, an antipyretic may be 7th birthday). -

CLINICAL TRIALS Safety and Immunogenicity of a Nicotine Conjugate Vaccine in Current Smokers
CLINICAL TRIALS Safety and immunogenicity of a nicotine conjugate vaccine in current smokers Immunotherapy is a novel potential treatment for nicotine addiction. The aim of this study was to assess the safety and immunogenicity of a nicotine conjugate vaccine, NicVAX, and its effects on smoking behavior. were recruited for a noncessation treatment study and assigned to 1 of 3 doses of the (68 ؍ Smokers (N nicotine vaccine (50, 100, or 200 g) or placebo. They were injected on days 0, 28, 56, and 182 and monitored for a period of 38 weeks. Results showed that the nicotine vaccine was safe and well tolerated. Vaccine immunogenicity was dose-related (P < .001), with the highest dose eliciting antibody concentrations within the anticipated range of efficacy. There was no evidence of compensatory smoking or precipitation of nicotine withdrawal with the nicotine vaccine. The 30-day abstinence rate was significantly different across with the highest rate of abstinence occurring with 200 g. The nicotine vaccine appears ,(02. ؍ the 4 doses (P to be a promising medication for tobacco dependence. (Clin Pharmacol Ther 2005;78:456-67.) Dorothy K. Hatsukami, PhD, Stephen Rennard, MD, Douglas Jorenby, PhD, Michael Fiore, MD, MPH, Joseph Koopmeiners, Arjen de Vos, MD, PhD, Gary Horwith, MD, and Paul R. Pentel, MD Minneapolis, Minn, Omaha, Neb, Madison, Wis, and Rockville, Md Surveys show that, although about 41% of smokers apy, is about 25% on average.2 Moreover, these per- make a quit attempt each year, less than 5% of smokers centages most likely exaggerate the efficacy of are successful at remaining abstinent for 3 months to a intervention because these trials are typically composed year.1 Smokers seeking available behavioral and phar- of subjects who are highly motivated to quit and who macologic therapies can enhance successful quit rates are free of complicating diagnoses such as depression 2 by 2- to 3-fold over control conditions. -

The Hepatitis B Vaccine Is the Most Widely Used Vaccine in the World, with Over 1 Billion Doses Given
Hepatitis B Vaccine Protect Yourself and Those You Love What is Hepatitis B? Hepatitis B is the most common serious liver infection in the world. It is caused by the hepatitis B virus (HBV), which attacks liver cells and can lead to liver failure, cirrhosis (scarring), or liver cancer later in life. The virus is transmitted through direct contact with infected blood and bodily fluids, and from an infected woman to her newborn at birth. Is there a safe vaccine for hepatitis B? YES! The good news is that there is a safe and effective vaccine for hepatitis B. The vaccine is a series, typically given as three shots over a six-month period that will provide a lifetime of protection. You cannot get hepatitis B from the vaccine – there is no human blood or live virus in the vaccine. The hepatitis B vaccine is the most widely used vaccine in the world, with over 1 billion doses given. The hepatitis B vaccine is the first "anti-cancer" vaccine because it can help prevent liver cancer! Who should be vaccinated against hepatitis B? The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) recommend the hepatitis B vaccine for all newborns and children up to 18 years of age, and all high-risk adults. All infants should receive the first dose of the vaccine in the delivery room or in the first 24 hours of life, preferably within 12 hours. (CDC recommends the first dose within 12 hours vs. the WHO recommendation of 24 hours.) The HBV vaccine is recommended to anyone who is at high risk of infection. -

Engerix-B Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME ENGERIX-B 20 micrograms/mL, suspension for injection. ENGERIX-B paediatric 10 micrograms/0.5 mL, suspension for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION ENGERIX-B paediatric dose: 10 microgram (µg) dose vaccine 1 dose (0.5 mL) contains: Hepatitis B surface antigen 1, 2 10 micrograms 1Adsorbed on aluminium hydroxide, hydrated Total: 0.25 milligrams Al3+ 2Produced in yeast cells (Saccharomyces cerevisiae) by recombinant DNA technology ENGERIX-B: 20 microgram (µg) dose vaccine 1 dose (1 mL) contains: Hepatitis B surface antigen1, 2 20 micrograms 1Adsorbed on aluminium hydroxide, hydrated Total: 0.50 milligrams Al3+ 2Produced in yeast cells (Saccharomyces cerevisiae) by recombinant DNA technology The vaccine is highly purified, and meets the WHO requirements for recombinant hepatitis B vaccines. No substances of human origin are used in its manufacture. For the full list of excipients, see section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Suspension for injection. ENGERIX-B is a turbid white suspension. Upon storage, a fine white deposit with a clear colourless supernatant may be observed. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications ENGERIX-B is indicated for active immunisation against hepatitis B virus infection. As part of the national immunisation schedule, the New Zealand Ministry of Health* recommend all infants, unvaccinated children up to the age of 16 years, and household and sexual contacts of known hepatitis B carriers receive a primary course of vaccination against hepatitis B. 1 Immunisation is also recommended for seronegative persons who are at substantial risk and have been demonstrated or judged to be susceptible to the hepatitis B virus (HBV). -
Diphtheria, Tetanus, Pertussis, Polio, Hib and Hepatitis B Vaccine for Babies and Children
Diphtheria, Tetanus, Pertussis, Polio, Hib and Hepatitis B vaccine for babies and children This leaflet tells you about the DTaP/IPV/Hib/ HepB vaccine, also known as “6 in 1” as it protects against six diseases, diphtheria, tetanus, pertussis (whooping cough), polio, Haemophilus influenzae type b and hepatitis B (HepB) disease. What does the vaccine protect against? Diphtheria Diphtheria is a serious disease that usually begins with a sore throat and can quickly cause breathing problems. It can damage the heart and nervous system and, in severe cases, can kill. Before diphtheria vaccine was introduced in the UK, there were up to 70,000 cases of diphtheria and around 5,000 deaths a year. Diphtheria can be spread from person to person through close contact. Tetanus Tetanus is a disease affecting the nervous system which can cause muscle spasms and breathing problems and can kill. It is caused when germs found in soil and manure get into the body through wounds or burns. Tetanus cannot be passed from person to person. 2 Published June 2018 Pertussis (whooping cough) Whooping cough is a disease that can cause long bouts of coughing and choking, making it hard to breathe. It can last for up to 10 weeks and babies under one year of age are most at risk. The disease is very serious and can kill. Before the pertussis vaccine was introduced, the average number of cases reported each year in the UK was 120,000 and 92 children died in the year before the vaccine was introduced. Whooping cough is usually spread by coughs and sneezes. -

Attenuation of Human Respiratory Syncytial Virus by Genome-Scale Codon-Pair Deoptimization
Attenuation of human respiratory syncytial virus by genome-scale codon-pair deoptimization Cyril Le Nouëna,1, Linda G. Brocka, Cindy Luongoa, Thomas McCartya, Lijuan Yanga, Masfique Mehedia, Eckard Wimmerb,1, Steffen Muellerb,2, Peter L. Collinsa, Ursula J. Buchholza,3, and Joshua M. DiNapolia,3,4 aRNA Viruses Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; and bDepartment of Molecular Genetics and Microbiology, Stony Brook University, Stony Brook, NY 11794 Contributed by Eckard Wimmer, June 18, 2014 (sent for review February 14, 2014) Human respiratory syncytial virus (RSV) is the most important viral acid coding is unaffected, CPD strains provide the same reper- agent of serious pediatric respiratory-tract disease worldwide. A toire of epitopes for inducing cellular and humoral immunity as vaccine or generally effective antiviral drug is not yet available. the WT pathogen. Recently, the CPD approach has been used We designed new live attenuated RSV vaccine candidates by successfully to attenuate poliovirus, influenza A virus, Strepto- codon-pair deoptimization (CPD). Specifically, viral ORFs were recoded coccus pneumonia, and HIV type 1 (5, 10–13). by rearranging existing synonymous codons to increase the content In the present work, four CPD RSV genomes were designed, of underrepresented codon pairs. Amino acid coding was com- synthesized, and recovered by reverse genetics. The CPD pletely unchanged. Four CPD RSV genomes were designed in recombinant (r) RSVs were attenuated and temperature- which the indicated ORFs were recoded: Min A (NS1, NS2, N, P, sensitive in vitro. Furthermore, we demonstrated that the CPD M, and SH), Min B (G and F), Min L (L), and Min FLC (all ORFs except rRSVs were attenuated and immunogenic in mice and African M2-1 and M2-2). -

Hepatitis B? HEPATITIS B Hepatitis B Is a Contagious Liver Disease That Results from Infection with the Hepatitis B Virus
What is Hepatitis B? HEPATITIS B Hepatitis B is a contagious liver disease that results from infection with the Hepatitis B virus. When first infected, a person can develop Are you at risk? an “acute” infection, which can range in severity from a very mild illness with few or no symptoms to a serious condition requiring hospitalization. Acute Hepatitis B refers to the first 6 months after someone is exposed to the Hepatitis B virus. Some people are able to fight the infection and clear the virus. For others, the infection remains and leads to a “chronic,” or lifelong, illness. Chronic Hepatitis B refers to the illness that occurs when the Hepatitis B virus remains in a person’s body. Over time, the infection can cause serious health problems. How is Hepatitis B spread? Hepatitis B is usually spread when blood, semen, or other body fluids from a person infected with the Hepatitis B virus enter the body of someone who is not infected. This can happen through having sex with an infected partner; sharing needles, syringes, or other injection drug equipment; or from direct contact with the blood or open sores of an infected person. Hepatitis B can also be passed from an infected mother to her baby at birth. Who should be tested for Hepatitis B? Approximately 1.2 million people in the United States and 350 million people worldwide have Hepatitis B. Testing for Hepatitis B is recommended for certain groups of people, including: Most are unaware of their infection. ■ People born in Asia, Africa, and other regions with moderate or high rates Is Hepatitis B common? of Hepatitis B (see map) Yes. -
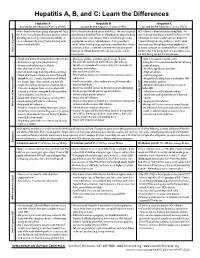
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Immunization Policies and Procedures Manual
Immunization Policies and Procedures Manual Louisiana Department of Health Office of Public Health Immunization Program Revised September 2017 i Center for Community and Preventive Health Bureau of Infectious Diseases Immunization Program TABLE OF CONTENTS I. POLICY AND GENERAL CLINIC POLICY ............................................................................................................................. 1 PURPOSE ........................................................................................................................................................................................... 1 POLICY ON CLINIC SCHEDULING ............................................................................................................................................ 2 POLICY ON PUBLICITY FOR IMMUNIZATION ACTIVITIES .............................................................................................. 4 POLICY ON EDUCATIONAL ACTIVITIES (HEALTH EDUCATION IN IMMUNIZATION CLINICS) .......................... 5 POLICY ON CHECKING IMMUNIZATION STATUS OF ALL CHILDREN RECEIVING SERVICES THROUGH THE HEALTH DEPARTMENT ...................................................................................................................................................... 6 POLICY ON MAXIMIZING TIME SPENT WITH PARENTS DURING IMMUNIZATION CLINICS ............................... 7 POLICY ON ASSISTANCE TO FOREIGN TRAVELERS .......................................................................................................... 9 II. POLICY -

UNICEF Immunization Roadmap 2018-2030
UNICEF IMMUNIZATION ROADMAP 2018–2030 Cover: ©UNICEF/UN065768/Khouder Al-Issa A health worker vaccinates 3-year-old Rahaf in Tareek Albab neighborhood in the eastern part of Aleppo city. UNICEF IMMUNIZATION ROADMAP 2018–2030 Photograph credits: Pages vi-vii: © Shutterstock/thi Page 5: © UNICEF/UN060913/Al-Issa Page 6: © UNICEF/UNI41364/Estey Page 10: © UNICEF/UN061432/Dejongh Page 14: © UNICEF/UNI76541/Holmes Page 18: © UNICEF/UN0125829/Sharma Page 24: © UNICEF/UN059884/Zar Mon Page 26: © UNICEF/UN065770/Al-Issa Page 33: © UNICEF/UN0143438/Alhariri Page 34: © UNICEF/UNI45693/Estey Page 38: © UNICEF/UNI77040/Holmes Page 40: © UNICEF/UN0125861/Sharma Page 44: © UNICEF/UNI41211/Holmes © United Nations Children’s Fund (UNICEF), September 2018 Permission is required to reproduce any part of this publication. Permission will be freely granted to educational or non-profit organizations. Please contact: Health Section, Immunization Team UNICEF 3 United Nations Plaza New York, NY 100017 Contents Abbreviations viii Executive summary 1 1. Introduction 7 1.1 Background 7 1.2 Developing the Roadmap 9 2. Key considerations informing the UNICEF Immunization Roadmap 11 2.1 Key drivers of immunization through 2030 11 2.2 Evolving immunization partnerships 15 2.3 UNICEF’s comparative advantage in immunization 16 3. What is new in this Roadmap? 19 3.1 Immunization coverage as a tracer indicator of child equity 19 3.2 Key areas of work 21 4. Roadmap programming framework 27 4.1 Vision and impact statements 27 4.2 Programming principles 27 4.3 Context-driven responses 27 4.4 Objectives and priorities 29 4.5 Populations and platforms 32 4.6 Country-level immunization strategies 32 5.