Attenuation of Human Respiratory Syncytial Virus by Genome-Scale Codon-Pair Deoptimization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children
March 19, 1999 / Vol. 48 / No. RR-2 Recommendations and Reports Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children Recommendations of the Advisory Committee on Immunization Practices (ACIP) Continuing Education Examination Education Continuing Inside: U.S. DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Disease Control and Prevention (CDC) Atlanta, Georgia 30333 The MMWR series of publications is published by the Epidemiology Program Office, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30333. SUGGESTED CITATION Centers for Disease Control and Prevention. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1999;48(No. RR-2):[inclu- sive page numbers]. Centers for Disease Control and Prevention....................Jeffrey P. Koplan, M.D., M.P.H. Director The material in this report was prepared for publication by National Center for Infectious Diseases.................................. James M. Hughes, M.D. Director Division of Viral and Rickettsial Diseases ................. Brian W. J. Mahy, Ph.D., Sc.D. Director National Immunization Program ...........................................Walter A. Orenstein, M.D. Director Epidemiology and Surveillance Division ...........................John R. Livengood, M.D. Director The production of this report as an MMWR serial publication was coordinated in Epidemiology Program Office.................................... Stephen B. Thacker, M.D., M.Sc. Director Office of Scientific and Health Communications ......................John W. Ward, M.D. Director Editor, MMWR Series Recommendations and Reports................................... Suzanne M. Hewitt, M.P.A. Managing Editor Valerie R. Johnson Project Editor Morie M. Higgins Visual Information Specialist Vol. 48 / No. RR-2 MMWR i Contents Clinical and Epidemiologic Features of Rotavirus Disease............................................. -

A Preliminary Study of Viral Metagenomics of French Bat Species in Contact with Humans: Identification of New Mammalian Viruses
A preliminary study of viral metagenomics of French bat species in contact with humans: identification of new mammalian viruses. Laurent Dacheux, Minerva Cervantes-Gonzalez, Ghislaine Guigon, Jean-Michel Thiberge, Mathias Vandenbogaert, Corinne Maufrais, Valérie Caro, Hervé Bourhy To cite this version: Laurent Dacheux, Minerva Cervantes-Gonzalez, Ghislaine Guigon, Jean-Michel Thiberge, Mathias Vandenbogaert, et al.. A preliminary study of viral metagenomics of French bat species in contact with humans: identification of new mammalian viruses.. PLoS ONE, Public Library of Science, 2014, 9 (1), pp.e87194. 10.1371/journal.pone.0087194.s006. pasteur-01430485 HAL Id: pasteur-01430485 https://hal-pasteur.archives-ouvertes.fr/pasteur-01430485 Submitted on 9 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License A Preliminary Study of Viral Metagenomics of French Bat Species in Contact with Humans: Identification of New Mammalian Viruses Laurent Dacheux1*, Minerva Cervantes-Gonzalez1, -

2020 Taxonomic Update for Phylum Negarnaviricota (Riboviria: Orthornavirae), Including the Large Orders Bunyavirales and Mononegavirales
Archives of Virology https://doi.org/10.1007/s00705-020-04731-2 VIROLOGY DIVISION NEWS 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales Jens H. Kuhn1 · Scott Adkins2 · Daniela Alioto3 · Sergey V. Alkhovsky4 · Gaya K. Amarasinghe5 · Simon J. Anthony6,7 · Tatjana Avšič‑Županc8 · María A. Ayllón9,10 · Justin Bahl11 · Anne Balkema‑Buschmann12 · Matthew J. Ballinger13 · Tomáš Bartonička14 · Christopher Basler15 · Sina Bavari16 · Martin Beer17 · Dennis A. Bente18 · Éric Bergeron19 · Brian H. Bird20 · Carol Blair21 · Kim R. Blasdell22 · Steven B. Bradfute23 · Rachel Breyta24 · Thomas Briese25 · Paul A. Brown26 · Ursula J. Buchholz27 · Michael J. Buchmeier28 · Alexander Bukreyev18,29 · Felicity Burt30 · Nihal Buzkan31 · Charles H. Calisher32 · Mengji Cao33,34 · Inmaculada Casas35 · John Chamberlain36 · Kartik Chandran37 · Rémi N. Charrel38 · Biao Chen39 · Michela Chiumenti40 · Il‑Ryong Choi41 · J. Christopher S. Clegg42 · Ian Crozier43 · John V. da Graça44 · Elena Dal Bó45 · Alberto M. R. Dávila46 · Juan Carlos de la Torre47 · Xavier de Lamballerie38 · Rik L. de Swart48 · Patrick L. Di Bello49 · Nicholas Di Paola50 · Francesco Di Serio40 · Ralf G. Dietzgen51 · Michele Digiaro52 · Valerian V. Dolja53 · Olga Dolnik54 · Michael A. Drebot55 · Jan Felix Drexler56 · Ralf Dürrwald57 · Lucie Dufkova58 · William G. Dundon59 · W. Paul Duprex60 · John M. Dye50 · Andrew J. Easton61 · Hideki Ebihara62 · Toufc Elbeaino63 · Koray Ergünay64 · Jorlan Fernandes195 · Anthony R. Fooks65 · Pierre B. H. Formenty66 · Leonie F. Forth17 · Ron A. M. Fouchier48 · Juliana Freitas‑Astúa67 · Selma Gago‑Zachert68,69 · George Fú Gāo70 · María Laura García71 · Adolfo García‑Sastre72 · Aura R. Garrison50 · Aiah Gbakima73 · Tracey Goldstein74 · Jean‑Paul J. Gonzalez75,76 · Anthony Grifths77 · Martin H. Groschup12 · Stephan Günther78 · Alexandro Guterres195 · Roy A. -

NIH Public Access Author Manuscript Arch Virol
NIH Public Access Author Manuscript Arch Virol. Author manuscript; available in PMC 2011 December 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Arch Virol. 2010 December ; 155(12): 2083±2103. doi:10.1007/s00705-010-0814-x. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations Jens H. Kuhn Integrated Research Facility at Fort Detrick (IRF-Frederick), Division of Clinical Research (DCR), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), National Interagency Biodefense Campus (NIBC), B-8200 Research Plaza, Fort Detrick, Frederick, MD 21702, USA Tunnell Consulting, Inc., King of Prussia, PA, USA Stephan Becker Institut für Virologie, Philipps-Universitaät Marburg, Marburg, Germany Hideki Ebihara Rocky Mountain Laboratories Integrated Research Facility, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT, USA Thomas W. Geisbert Galveston National Laboratory, University of Texas Medical Branch, Galveston, TX, USA Karl M. Johnson University of New Mexico, Albuquerque, NM, USA Yoshihiro Kawaoka School of Veterinary Medicine, University of Wisconsin, Madison, WI, USA W. Ian Lipkin Center for Infection and Immunity, Columbia University Medical Center, New York, NY, USA Ana I. Negredo Centro Nacional de Microbiología, Instituto de Salud Carlos III, Madrid, Spain Sergey V. Netesov Novosibirsk State University, Novosibirsk, Novosibirsk Oblast, Russia Stuart T. Nichol Centers for Disease Control and Prevention, Atlanta, GA, USA Gustavo Palacios Center for Infection and Immunity, Columbia University Medical Center, New York, NY, USA Clarence J. Peters © Springer-Verlag (outside the USA) 2010 [email protected] . -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful. -

Wastewater Based Epidemiology Perspective As a Faster Protocol for Detecting Coronavirus RNA in Human Populations: a Review with Specific Reference to SARS-Cov-2 Virus
pathogens Review Wastewater Based Epidemiology Perspective as a Faster Protocol for Detecting Coronavirus RNA in Human Populations: A Review with Specific Reference to SARS-CoV-2 Virus Milad Mousazadeh 1,2,†,‡ , Razieh Ashoori 3, Biswaranjan Paital 4 , I¸sıkKabda¸slı 5,‡ , Zacharias Frontistis 6 , Marjan Hashemi 7 , Miguel A. Sandoval 8,9,‡ , Samendra Sherchan 10, Kabita Das 11 and Mohammad Mahdi Emamjomeh 12,* 1 Student Research Committee, Qazvin University of Medical Sciences, Qazvin, Iran; [email protected] 2 Department of Environmental Health Engineering, School of Health, Qazvin University of Medical Sciences, Qazvin, Iran 3 Department of Environmental Health Engineering, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran; [email protected] 4 Redox Regulation Laboratory, College of Basic Science and Humanities, Odisha University of Agriculture and Technology, Bhubaneswar 751003, India; [email protected] 5 Environmental Engineering Department, Civil Engineering Faculty, Ayaza˘gaCampus, Istanbul˙ Technical University, Istanbul˙ 34469, Turkey; [email protected] 6 Department of Chemical Engineering, University of Western Macedonia, 50132 Kozani, Greece; Citation: Mousazadeh, M.; Ashoori, [email protected] 7 Environmental and Occupational Hazards Control Research Center, Shahid Beheshti University of Medical R.; Paital, B.; Kabda¸slı,I.; Frontistis, Sciences, Tehran, Iran; [email protected] Z.; Hashemi, M.; Sandoval, M.A.; 8 Laboratorio de Electroquímica Medio Ambiental LEQMA, Departamento -

Preventing Polio WHO Myanmar Newsletter Special , 5 August 2019
Preventing polio WHO Myanmar newsletter special , 5 August 2019 What is polio? Poliomyelitis (polio) is a highly infectious disease that is caused when the polio virus invades the nervous system of an infected person. Polio can cause paralysis and even death. Who is most at risk for polio? Poliovirus usually affects children under 5 years of age who are unvaccinated or under-vaccinated. The virus can also affect or be carried by adolescents and adults. photo credit: Ms Lei Lei Mon, WHO Myanmar The child or individual suspected of polio may A child receives oral polio vaccine at Thandaung township, complain of sudden onset of weakness of arms Kayin State, 21 July 2019 and/or legs. Oral polio vaccine is the vaccine Is there a cure for polio? recommended for polio eradication Can it be prevented? There is no cure for polio. The disease can severely paralyze, or even kill, an infected child. Is the polio vaccine safe? Oral polio vaccine is one of the safest vaccines Polio can be prevented by immunizing a child with ever developed. It is so safe it can be given to sick approrpiate vaccination. There are currently two children and newborns. Since 1961, when oral effective polio vaccines, the inactivated poliovirus polio vaccine was introduced, two billion children vaccine (IPV) and the live attenuated oral polio have been immunized against polio globally. vaccine (OPV). This is estimated to have saved at least 16 million children from permanent paralysis by polio, world- wide. It is also safe to administer multiple doses of polio vaccine to children. -

A Novel Ebola Virus VP40 Matrix Protein-Based Screening for Identification of Novel Candidate Medical Countermeasures
viruses Communication A Novel Ebola Virus VP40 Matrix Protein-Based Screening for Identification of Novel Candidate Medical Countermeasures Ryan P. Bennett 1,† , Courtney L. Finch 2,† , Elena N. Postnikova 2 , Ryan A. Stewart 1, Yingyun Cai 2 , Shuiqing Yu 2 , Janie Liang 2, Julie Dyall 2 , Jason D. Salter 1 , Harold C. Smith 1,* and Jens H. Kuhn 2,* 1 OyaGen, Inc., 77 Ridgeland Road, Rochester, NY 14623, USA; [email protected] (R.P.B.); [email protected] (R.A.S.); [email protected] (J.D.S.) 2 NIH/NIAID/DCR/Integrated Research Facility at Fort Detrick (IRF-Frederick), Frederick, MD 21702, USA; courtney.fi[email protected] (C.L.F.); [email protected] (E.N.P.); [email protected] (Y.C.); [email protected] (S.Y.); [email protected] (J.L.); [email protected] (J.D.) * Correspondence: [email protected] (H.C.S.); [email protected] (J.H.K.); Tel.: +1-585-697-4351 (H.C.S.); +1-301-631-7245 (J.H.K.) † These authors contributed equally to this work. Abstract: Filoviruses, such as Ebola virus and Marburg virus, are of significant human health concern. From 2013 to 2016, Ebola virus caused 11,323 fatalities in Western Africa. Since 2018, two Ebola virus disease outbreaks in the Democratic Republic of the Congo resulted in 2354 fatalities. Although there is progress in medical countermeasure (MCM) development (in particular, vaccines and antibody- based therapeutics), the need for efficacious small-molecule therapeutics remains unmet. Here we describe a novel high-throughput screening assay to identify inhibitors of Ebola virus VP40 matrix protein association with viral particle assembly sites on the interior of the host cell plasma membrane. -

2021 Medicare Vaccine Coverage Part B Vs Part D
CDPHP® Medicare Advantage Vaccine Coverage Guide Part B (Medical) vs. Part D (Pharmacy) Medicare Part B (Medical): Medicare Part D (Pharmacy): Vaccinations or inoculations Vaccinations or inoculations are included when the administration is (except influenza, pneumococcal, reasonable and necessary for the prevention of illness. and hepatitis B for members at risk) are excluded unless they are directly related to the treatment of an injury or direct exposure to a disease or condition. • Influenza Vaccine (Flu) • BCG Vaccine • Pneumococcal Vaccine • Diphtheria/Tetanus/Acellular Pertussis Vaccine (ADACEL, (Pneumovax, Prevnar 13) BOOSTRIX, DAPTACEL, INFANRIX) • Hepatitis B Vaccine • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus (Recombivax, Engerix-B) Vaccine (KINRIX, QUADRACEL) for members at moderate • Diphtheria/Tetanus Vaccine (DT, Td, TDVAX, TENIVAC) to high risk • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus Vac • Other vaccines when directly cine/ Haemophilus Influenzae Type B Conjugate Vaccine (PENTACEL) related to the treatment of an • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus injury or direct exposure to a Vaccine/Hepatitis B Vaccine (PEDIARIX) disease or condition, such as: • Haemophilus Influenzae Type B Conjugate Vaccine (ActHIB, PedvaxHIB, • Antivenom Sera Hiberix) • Diphtheria/Tetanus Vaccine • Hepatitis A Vaccine, Inactivated (VAQTA) (DT, Td, TDVAX, TENIVAC) • Hepatitis B Vaccine, Recombinant (ENGERIX-B, RECOMBIVAX HB) • Rabies Virus Vaccine for members at low risk (RabAvert, -

IRES Knocks out Rabies
RESEARCH HIGHLIGHTS VIRAL PATHOGENESIS IRES knocks out rabies Non-segmented negative-strand from poliovirus or human rhinovirus Infection of neurons with RNA RNA viruses that can infect neurons type 2 (HRV2) enables downregula- viruses is initially controlled by in the central nervous system include tion of phosphoprotein expression interferon-β (IFNβ) and subsequently measles virus, mumps virus, Borna at the level of translation and can by virus-specific antibodies and virus, the emerging deadly Nipah render rabies virus avirulent. T-cell derived IFNβ. The rabies virus and Hendra viruses and rabies virus. Although rabies and other phosphoprotein, which is an essential Until now there has been no method non-segmented negative-strand cofactor of the viral polymerase and available to control the expression RNA viruses can replicate in all also has a role in encapsidation of of individual genes of these viruses. cell types, positive-strand RNA replicated genomes, functions to According to a report published in viruses, such as poliovirus and dampen the host response by coun- the Journal of Virology, replacing the HRV2, have a restricted host range. teracting the production of IFN. The transcription signals of the rabies There is evidence that IRESs in the most striking finding from this study virus phosphoprotein gene with 5′-untranslated regions of positive- is that translational attenuation of internal ribosome entry sites (IRESs) strand RNA viruses are subject to phosphoprotein through insertion of cell-type inhibition and can mediate IRESs from poliovirus or HRV2 lim- cell-type specificity. To test whether ited the ability of recombinant rabies Corbis IRESs can affect gene expression of viruses to counteract the production non-segmented negative-strand RNA of IFN, as shown by the failure of viruses, Marschalek et al. -
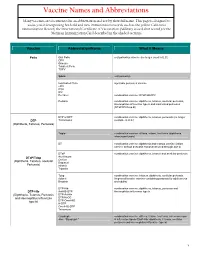
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

A Bioinformatic Analysis of the Mononegavirales
A BIOINFORMATIC ANALYSIS OF THE MONONEGAVIRALES TRANSCRIPTION/REPLICATION COMPLEX THROUGH THE DEVELOPMENT OF THE DISSIC PIPELINE by Sean Bruce Cleveland A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Microbiology MONTANA STATE UNIVERSITY Bozeman, Montana April, 2013 ©COPYRIGHT by Sean Bruce Cleveland 2013 All Rights Reserved ii APPROVAL of a dissertation submitted by Sean Bruce Cleveland This dissertation has been read by each member of the dissertation committee and has been found to be satisfactory regarding content, English usage, format, citation, bibliographic style, and consistency and is ready for submission to The Graduate School. Marcella A. McClure Approved for the Department of Microbiology Mark Jutila Approved for The Graduate School Dr. Ronald W. Larsen iii STATEMENT OF PERMISSION TO USE In presenting this dissertation in partial fulfillment of the requirements for a doctoral degree at Montana State University, I agree that the Library shall make it available to borrowers under rules of the Library. I further agree that copying of this dissertation is allowable only for scholarly purposes, consistent with “fair use” as prescribed in the U.S. Copyright Law. Requests for extensive copying or reproduction of this dissertation should be referred to ProQuest Information and Learning, 300 North Zeeb Road, Ann Arbor, Michigan 48106, to whom I have granted “the exclusive right to reproduce and distribute my dissertation in and from microform along with the non- exclusive right to reproduce and distribute my abstract in any format in whole or in part.” Sean Bruce Cleveland April 2013 iv DEDICATION I dedicate this dissertation to my fiancé Jessica and my mother Shelby for their undying support and understanding all these years.