Wastewater Based Epidemiology Perspective As a Faster Protocol for Detecting Coronavirus RNA in Human Populations: a Review with Specific Reference to SARS-Cov-2 Virus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children
March 19, 1999 / Vol. 48 / No. RR-2 Recommendations and Reports Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children Recommendations of the Advisory Committee on Immunization Practices (ACIP) Continuing Education Examination Education Continuing Inside: U.S. DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Disease Control and Prevention (CDC) Atlanta, Georgia 30333 The MMWR series of publications is published by the Epidemiology Program Office, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30333. SUGGESTED CITATION Centers for Disease Control and Prevention. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1999;48(No. RR-2):[inclu- sive page numbers]. Centers for Disease Control and Prevention....................Jeffrey P. Koplan, M.D., M.P.H. Director The material in this report was prepared for publication by National Center for Infectious Diseases.................................. James M. Hughes, M.D. Director Division of Viral and Rickettsial Diseases ................. Brian W. J. Mahy, Ph.D., Sc.D. Director National Immunization Program ...........................................Walter A. Orenstein, M.D. Director Epidemiology and Surveillance Division ...........................John R. Livengood, M.D. Director The production of this report as an MMWR serial publication was coordinated in Epidemiology Program Office.................................... Stephen B. Thacker, M.D., M.Sc. Director Office of Scientific and Health Communications ......................John W. Ward, M.D. Director Editor, MMWR Series Recommendations and Reports................................... Suzanne M. Hewitt, M.P.A. Managing Editor Valerie R. Johnson Project Editor Morie M. Higgins Visual Information Specialist Vol. 48 / No. RR-2 MMWR i Contents Clinical and Epidemiologic Features of Rotavirus Disease............................................. -

Cov-2 Wastewater Surveillance System (Vatar COVID-19) from December 2020 to March
medRxiv preprint doi: https://doi.org/10.1101/2021.05.27.21257918; this version posted May 30, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Monitoring emergence of SARS-CoV-2 B.1.1.7 Variant through the Spanish National SARS- CoV-2 Wastewater Surveillance System (VATar COVID-19) from December 2020 to March 2021 Albert Carcereny1, Adán Martínez-Velázquez1, Albert Bosch1, Ana Allende2, Pilar Truchado2, Jenifer Cascales2, Jesús L Romalde3, Marta Lois3, David Polo3, Gloria Sánchez4, Alba Pérez- Cataluña4, Azahara Díaz-Reolid4, Andrés Antón5, Josep Gregori6,7, Damir Garcia-Cehic6,7, Josep Quer6,7, Margarita Palau8, Cristina González Ruano9, Rosa M Pintó1#* and Susana Guix1#* 1 Enteric Virus laboratory, Department of Genetics, Microbiology and Statistics, Section of Microbiology, Virology and Biotechnology, School of Biology, and Institute of Nutrition and Food Safety (INSA), University of Barcelona, Barcelona, Spain. 2 Research Group on Microbiology and Quality of Fruit and Vegetables, CEBAS-CSIC, Campus Universitario de Espinardo, 25, 30100, Murcia, Spain. 3 Department of Microbiology and Parasitology, CIBUS-Faculty of Biology & Institute CRETUS, Universidade de Santiago de Compostela, Santiago de Compostela, 15782, Spain. 4 Department of Preservation and Food Safety Technologies, Institute of Agrochemistry and Food Technology, IATA-CSIC, Av. Agustín Escardino 7, Paterna, 46980, Valencia, Spain. 5 Microbiology Department, Vall d'Hebron Institut de Recerca (VHIR), Vall d'Hebron Barcelona Hospital Campus, Passeig Vall d'Hebron 119-129, 08035 Barcelona, Spain. -

Attenuation of Human Respiratory Syncytial Virus by Genome-Scale Codon-Pair Deoptimization
Attenuation of human respiratory syncytial virus by genome-scale codon-pair deoptimization Cyril Le Nouëna,1, Linda G. Brocka, Cindy Luongoa, Thomas McCartya, Lijuan Yanga, Masfique Mehedia, Eckard Wimmerb,1, Steffen Muellerb,2, Peter L. Collinsa, Ursula J. Buchholza,3, and Joshua M. DiNapolia,3,4 aRNA Viruses Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; and bDepartment of Molecular Genetics and Microbiology, Stony Brook University, Stony Brook, NY 11794 Contributed by Eckard Wimmer, June 18, 2014 (sent for review February 14, 2014) Human respiratory syncytial virus (RSV) is the most important viral acid coding is unaffected, CPD strains provide the same reper- agent of serious pediatric respiratory-tract disease worldwide. A toire of epitopes for inducing cellular and humoral immunity as vaccine or generally effective antiviral drug is not yet available. the WT pathogen. Recently, the CPD approach has been used We designed new live attenuated RSV vaccine candidates by successfully to attenuate poliovirus, influenza A virus, Strepto- codon-pair deoptimization (CPD). Specifically, viral ORFs were recoded coccus pneumonia, and HIV type 1 (5, 10–13). by rearranging existing synonymous codons to increase the content In the present work, four CPD RSV genomes were designed, of underrepresented codon pairs. Amino acid coding was com- synthesized, and recovered by reverse genetics. The CPD pletely unchanged. Four CPD RSV genomes were designed in recombinant (r) RSVs were attenuated and temperature- which the indicated ORFs were recoded: Min A (NS1, NS2, N, P, sensitive in vitro. Furthermore, we demonstrated that the CPD M, and SH), Min B (G and F), Min L (L), and Min FLC (all ORFs except rRSVs were attenuated and immunogenic in mice and African M2-1 and M2-2). -

Standards to Support an Enduring Capability in Wastewater Surveillance for Public Health
DHS/NIST Workshop: Standards to Support an Enduring Capability in Wastewater Surveillance for Public Health Poster Abstracts June 14-18th 2021 Virtual Poster Session https://swwsworkshop.virtualpostersession.org/ CEDAR-MC: Clinical and Environmental Dynamics of Antibiotic Resistance within Microbial Communities George Hanna1,2, Bashir Hamidi1, Scott Curry1, Cheryl Carmack2, Alexander V. Alekseyenko1 1Medical University of South Carolina; 2Charleston Waterkeeper Introduction: Escape into the environment and the persistence of antibiotic resistance is an imminent threat to the healthcare advances attained in the 20th century. Microbial communities that co-exist with resistant bacteria may help uncover novel strategies for global antimicrobial control and curb emergence and maintenance of resistance. However, availability of clinically relevant specimens with complementary samples from the built and natural environment is a major obstacle to effective studies of the dynamics of resistance in the affected human populations and in their surroundings. Methods: We bring together environmental and clinical measurements of the microbial communities with evidence for emerging resistance by linking existing local clinical and environmental surveillance programs. The clinical specimens are sourced from the Medical University of South Carolina (MUSC) infection surveillance culture program that routinely samples the MUSC patient population for clinically relevant pathogens. The environmental specimens are the result of partnership with a local non-profit, -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful. -

Preventing Polio WHO Myanmar Newsletter Special , 5 August 2019
Preventing polio WHO Myanmar newsletter special , 5 August 2019 What is polio? Poliomyelitis (polio) is a highly infectious disease that is caused when the polio virus invades the nervous system of an infected person. Polio can cause paralysis and even death. Who is most at risk for polio? Poliovirus usually affects children under 5 years of age who are unvaccinated or under-vaccinated. The virus can also affect or be carried by adolescents and adults. photo credit: Ms Lei Lei Mon, WHO Myanmar The child or individual suspected of polio may A child receives oral polio vaccine at Thandaung township, complain of sudden onset of weakness of arms Kayin State, 21 July 2019 and/or legs. Oral polio vaccine is the vaccine Is there a cure for polio? recommended for polio eradication Can it be prevented? There is no cure for polio. The disease can severely paralyze, or even kill, an infected child. Is the polio vaccine safe? Oral polio vaccine is one of the safest vaccines Polio can be prevented by immunizing a child with ever developed. It is so safe it can be given to sick approrpiate vaccination. There are currently two children and newborns. Since 1961, when oral effective polio vaccines, the inactivated poliovirus polio vaccine was introduced, two billion children vaccine (IPV) and the live attenuated oral polio have been immunized against polio globally. vaccine (OPV). This is estimated to have saved at least 16 million children from permanent paralysis by polio, world- wide. It is also safe to administer multiple doses of polio vaccine to children. -

Wastewater Surveillance Collaborative MOU FINAL
Colorado SARS-CoV-2 Wastewater Surveillance Collaborative Memorandum of Understanding July 2020 Section 1 Background The Colorado Department of Public Health and Environment (Department) is partnering with Colorado State University, Metropolitan State University of Denver (MSU), and 17 Wastewater Utilities to develop a statewide wastewater surveillance system of SARS-CoV-2 RNA, the etiological agent of COVID19. The purpose of the surveillance system is to provide early warning (days to a week) for state and local health authorities of significant changes in fecal shedding of SARS-CoV-2 that could be used in combination with other surveillance efforts and drive action in responding to future COVID19 outbreaks. This testing could also confirm downward trends in COVID-19 outbreaks. With more data and analysis, it may also be useful for predicting community prevalence or to identify potential virus hot spots. Section 2 Purpose This agreement is being entered into by the Memorandum of Understanding (MOU) parties so that roles and responsibilities of each member of the collaborative are understood. The Department has secured $520,000 in funding for this project and this MOU is focused on how those dollars will be leveraged. The effort is scalable and may be expanded through future agreements. The financial obligations under this MOU are contingent upon appropriation, budgeting, and availability of specific funds to discharge those obligations. Nothing in this MOU constitutes a debt, a direct or indirect multiple fiscal year financial obligation or a pledge of a Wastewater Utilities’ credit. Section 3 MOU Parties The following entities are parties to this MOU, individually referred to as Wastewater Utilities, Universities, and Regulatory Agencies. -

2021 Medicare Vaccine Coverage Part B Vs Part D
CDPHP® Medicare Advantage Vaccine Coverage Guide Part B (Medical) vs. Part D (Pharmacy) Medicare Part B (Medical): Medicare Part D (Pharmacy): Vaccinations or inoculations Vaccinations or inoculations are included when the administration is (except influenza, pneumococcal, reasonable and necessary for the prevention of illness. and hepatitis B for members at risk) are excluded unless they are directly related to the treatment of an injury or direct exposure to a disease or condition. • Influenza Vaccine (Flu) • BCG Vaccine • Pneumococcal Vaccine • Diphtheria/Tetanus/Acellular Pertussis Vaccine (ADACEL, (Pneumovax, Prevnar 13) BOOSTRIX, DAPTACEL, INFANRIX) • Hepatitis B Vaccine • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus (Recombivax, Engerix-B) Vaccine (KINRIX, QUADRACEL) for members at moderate • Diphtheria/Tetanus Vaccine (DT, Td, TDVAX, TENIVAC) to high risk • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus Vac • Other vaccines when directly cine/ Haemophilus Influenzae Type B Conjugate Vaccine (PENTACEL) related to the treatment of an • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus injury or direct exposure to a Vaccine/Hepatitis B Vaccine (PEDIARIX) disease or condition, such as: • Haemophilus Influenzae Type B Conjugate Vaccine (ActHIB, PedvaxHIB, • Antivenom Sera Hiberix) • Diphtheria/Tetanus Vaccine • Hepatitis A Vaccine, Inactivated (VAQTA) (DT, Td, TDVAX, TENIVAC) • Hepatitis B Vaccine, Recombinant (ENGERIX-B, RECOMBIVAX HB) • Rabies Virus Vaccine for members at low risk (RabAvert, -

IRES Knocks out Rabies
RESEARCH HIGHLIGHTS VIRAL PATHOGENESIS IRES knocks out rabies Non-segmented negative-strand from poliovirus or human rhinovirus Infection of neurons with RNA RNA viruses that can infect neurons type 2 (HRV2) enables downregula- viruses is initially controlled by in the central nervous system include tion of phosphoprotein expression interferon-β (IFNβ) and subsequently measles virus, mumps virus, Borna at the level of translation and can by virus-specific antibodies and virus, the emerging deadly Nipah render rabies virus avirulent. T-cell derived IFNβ. The rabies virus and Hendra viruses and rabies virus. Although rabies and other phosphoprotein, which is an essential Until now there has been no method non-segmented negative-strand cofactor of the viral polymerase and available to control the expression RNA viruses can replicate in all also has a role in encapsidation of of individual genes of these viruses. cell types, positive-strand RNA replicated genomes, functions to According to a report published in viruses, such as poliovirus and dampen the host response by coun- the Journal of Virology, replacing the HRV2, have a restricted host range. teracting the production of IFN. The transcription signals of the rabies There is evidence that IRESs in the most striking finding from this study virus phosphoprotein gene with 5′-untranslated regions of positive- is that translational attenuation of internal ribosome entry sites (IRESs) strand RNA viruses are subject to phosphoprotein through insertion of cell-type inhibition and can mediate IRESs from poliovirus or HRV2 lim- cell-type specificity. To test whether ited the ability of recombinant rabies Corbis IRESs can affect gene expression of viruses to counteract the production non-segmented negative-strand RNA of IFN, as shown by the failure of viruses, Marschalek et al. -
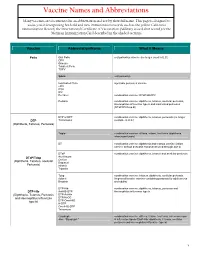
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Media Monitoring: Extract of Press News on Higher Education in Africa
Issue 86 Media Monitoring: Extract of Press News on Higher Education in Africa 1. World Bank Increased Investment in Zimbabwe’s Tertiary Education Essential to Economic Growth, Human Capital Development (Zimbabwe) Extensive reforms are required to translate the government’s education vision into a concrete set of programs and projects to accelerate economic recovery and reduce socioeconomic disparities, the Zimbabwe Higher and Tertiary Education Sector Analysis Report found. Developed by the World Bank and the Ministry of Higher and Tertiary Education, Innovation, Science and Technology Department (MHTEISTD), the report acknowledges the ongoing reform efforts that the department has embarked on under its Education 5.0 strategy to revitalize higher and tertiary education through the five pillars of Teaching, Research, Community Service, Innovation, and Industrialization. The report also finds that throughout the past decade, Zimbabwe has sustained a high level of public education spending, including spending on tertiary education, relative to the size of its economy. The macro-economic challenges in that last two years have however seen significant decline in education spending both as a percentage of total government expenditure and as a percentage of gross domestic product. “The government’s longstanding commitment to education spending reflects the importance of human-capital development as a national cultural value. As we are fully cognizant of the ever-changing world in which we operate we now seek to transform our Tertiary Education to meaningfully impact economic productivity and workforce skills development,” said Professor Fanuel Tagwira, Permanent Secretary, MHTIESTD. Th education analysis underscores the recent World Bank Digital Economy Diagnostic for Zimbabwe launched in May, which revealed that Zimbabwe is facing a significant skills deficit in science and technology-linked job roles, including digital skills. -

Global COVID-19 Wastewater Monitoring Efforts, Equity, and Gaps
medRxiv preprint doi: https://doi.org/10.1101/2021.03.14.21253564; this version posted March 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Show us the Data: Global COVID-19 Wastewater Monitoring Efforts, Equity, and Gaps Colleen C. Naughton*1, Fernando A. Roman, Jr.1, Ana Grace F. Alvarado1, Arianna Q. Tariqi1, Matthew A. Deeming1, Kyle Bibby2, Aaron Bivins2, Joan B. Rose3, Gertjan Medema456, Warish Ahmed7, Panagis Katsivelis8, Vajra Allan9, Ryan Sinclair10, Yihan Zhang11, Maureen N. Kinyua11 *Corresponding Author [email protected] 1Department of Civil and Environmental Engineering, University of California at Merced, Merced, CA 95343, USA 2Department of Civil & Environmental Engineering & Earth Science, University of Notre Dame, 156 FitZpatrick Hall, Notre Dame, IN, 46556, USA. 3Department of Fisheries and Wildlife, Michigan State University, East Lansing, Michigan 48824, USA 4KWR Water Research Institute, Groningenhaven 7, Nieuwegein, 3433 PE, the Netherlands 4Delft University of Technology, Stevinweg 1, Delft, 2628 CN, the Netherlands 6Michigan State University, 1405 S Harrison Rd, East-Lansing, Michigan, 48823, USA 7CSIRO Land and Water, Ecosciences Precinct, 41 Boggo Road, QLD 4102, Australia. 8Venthic Technologies, Kipoupoleos 129, Peristeri, Athens, Greece 9PATH 2201Westlake Avenue, Suite 200 Seattle, WA 98121, USA 10Schools of Public Health and Earth and Biological Sciences, Loma Linda University Loma Linda, CA 92350, USA 11Department of Civil and Environmental Engineering, University of California at Davis, Davis, CA 95616, USA Abstract (200 words) A year since the declaration of the global coronavirus disease 2019 (COVID-19) pandemic there have been over 110 million cases and 2.5 million deaths.