Enteric Hepatitis Viruses1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children
March 19, 1999 / Vol. 48 / No. RR-2 Recommendations and Reports Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children Recommendations of the Advisory Committee on Immunization Practices (ACIP) Continuing Education Examination Education Continuing Inside: U.S. DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Disease Control and Prevention (CDC) Atlanta, Georgia 30333 The MMWR series of publications is published by the Epidemiology Program Office, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30333. SUGGESTED CITATION Centers for Disease Control and Prevention. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1999;48(No. RR-2):[inclu- sive page numbers]. Centers for Disease Control and Prevention....................Jeffrey P. Koplan, M.D., M.P.H. Director The material in this report was prepared for publication by National Center for Infectious Diseases.................................. James M. Hughes, M.D. Director Division of Viral and Rickettsial Diseases ................. Brian W. J. Mahy, Ph.D., Sc.D. Director National Immunization Program ...........................................Walter A. Orenstein, M.D. Director Epidemiology and Surveillance Division ...........................John R. Livengood, M.D. Director The production of this report as an MMWR serial publication was coordinated in Epidemiology Program Office.................................... Stephen B. Thacker, M.D., M.Sc. Director Office of Scientific and Health Communications ......................John W. Ward, M.D. Director Editor, MMWR Series Recommendations and Reports................................... Suzanne M. Hewitt, M.P.A. Managing Editor Valerie R. Johnson Project Editor Morie M. Higgins Visual Information Specialist Vol. 48 / No. RR-2 MMWR i Contents Clinical and Epidemiologic Features of Rotavirus Disease............................................. -
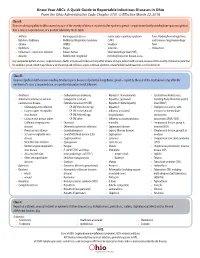
The Know Your Abcs: a Quick Guide to Reportable Infectious Diseases
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective March 22, 2018 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Meningococcal disease • Severe acute respiratory syndrome fever, Marburg hemorrhagic fever, • Botulism, foodborne • Middle East Respiratory Syndrome (SARS) and Crimean-Congo hemorrhagic • Cholera (MERS) • Smallpox fever • Diphtheria • Plague • Tularemia • Yellow fever • Influenza A – novel virus infection • Rabies, human • Viral hemorrhagic fever (VHF), • Measles • Rubella (not congenital) including Ebola virus disease, Lassa Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis C (non-perinatal) • Spotted Fever Rickettsiosis, • Arboviral neuroinvasive and non- carbapenem-resistant • Hepatitis C (perinatal) including Rocky Mountain spotted neuroinvasive disease: Enterobacteriaceae (CP-CRE) • Hepatitis D (delta hepatitis) fever (RMSF) • Chikungunya virus infection • CP-CRE Enterobacter spp. • Hepatitis E • Staphylococcus aureus, with • Eastern equine encephalitis • CP-CRE Escherichia coli • Influenza-associated resistance or intermediate virus disease • CP-CRE Klebsiella spp. -

Reportable Diseases and Conditions
KINGS COUNTY DEPARTMENT of PUBLIC HEALTH 330 CAMPUS DRIVE, HANFORD, CA 93230 REPORTABLE DISEASES AND CONDITIONS Title 17, California Code of Regulations, §2500, requires that known or suspected cases of any of the diseases or conditions listed below are to be reported to the local health jurisdiction within the specified time frame: REPORT IMMEDIATELY BY PHONE During Business Hours: (559) 852-2579 After Hours: (559) 852-2720 for Immediate Reportable Disease and Conditions Anthrax Escherichia coli: Shiga Toxin producing (STEC), Rabies (Specify Human or Animal) Botulism (Specify Infant, Foodborne, Wound, Other) including E. coli O157:H7 Scrombroid Fish Poisoning Brucellosis, Human Flavivirus Infection of Undetermined Species Shiga Toxin (Detected in Feces) Cholera Foodborne Disease (2 or More Cases) Smallpox (Variola) Ciguatera Fish Poisoning Hemolytic Uremic Syndrome Tularemia, human Dengue Virus Infection Influenza, Novel Strains, Human Viral Hemorrhagic Fever (Crimean-Congo, Ebola, Diphtheria Measles (Rubeola) Lassa, and Marburg Viruses) Domonic Acid Poisoning (Amnesic Shellfish Meningococcal Infections Yellow Fever Poisoning) Novel Virus Infection with Pandemic Potential Zika Virus Infection Paralytic Shellfish Poisoning Plague (Specify Human or Animal) Immediately report the occurrence of any unusual disease OR outbreaks of any disease. REPORT BY PHONE, FAX, MAIL WITHIN ONE (1) WORKING DAY Phone: (559) 852-2579 Fax: (559) 589-0482 Mail: 330 Campus Drive, Hanford 93230 Conditions may also be reported electronically via the California -

Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

Attenuation of Human Respiratory Syncytial Virus by Genome-Scale Codon-Pair Deoptimization
Attenuation of human respiratory syncytial virus by genome-scale codon-pair deoptimization Cyril Le Nouëna,1, Linda G. Brocka, Cindy Luongoa, Thomas McCartya, Lijuan Yanga, Masfique Mehedia, Eckard Wimmerb,1, Steffen Muellerb,2, Peter L. Collinsa, Ursula J. Buchholza,3, and Joshua M. DiNapolia,3,4 aRNA Viruses Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; and bDepartment of Molecular Genetics and Microbiology, Stony Brook University, Stony Brook, NY 11794 Contributed by Eckard Wimmer, June 18, 2014 (sent for review February 14, 2014) Human respiratory syncytial virus (RSV) is the most important viral acid coding is unaffected, CPD strains provide the same reper- agent of serious pediatric respiratory-tract disease worldwide. A toire of epitopes for inducing cellular and humoral immunity as vaccine or generally effective antiviral drug is not yet available. the WT pathogen. Recently, the CPD approach has been used We designed new live attenuated RSV vaccine candidates by successfully to attenuate poliovirus, influenza A virus, Strepto- codon-pair deoptimization (CPD). Specifically, viral ORFs were recoded coccus pneumonia, and HIV type 1 (5, 10–13). by rearranging existing synonymous codons to increase the content In the present work, four CPD RSV genomes were designed, of underrepresented codon pairs. Amino acid coding was com- synthesized, and recovered by reverse genetics. The CPD pletely unchanged. Four CPD RSV genomes were designed in recombinant (r) RSVs were attenuated and temperature- which the indicated ORFs were recoded: Min A (NS1, NS2, N, P, sensitive in vitro. Furthermore, we demonstrated that the CPD M, and SH), Min B (G and F), Min L (L), and Min FLC (all ORFs except rRSVs were attenuated and immunogenic in mice and African M2-1 and M2-2). -

Novel Hepatitis D-Like Agents in Vertebrates and Invertebrates
bioRxiv preprint doi: https://doi.org/10.1101/539924; this version posted February 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Novel hepatitis D-like agents in vertebrates and invertebrates 2 3 4 Wei-Shan Chang1, John H.-O. Pettersson1, Callum Le Lay1, Mang Shi1, Nathan Lo1, Michelle 5 Wille2, John-Sebastian Eden1,3, Edward C. Holmes1 6 7 8 1Marie Bashir Institute for Infectious Diseases and Biosecurity, Charles Perkins Centre, 9 School of Life and Environmental Sciences and Sydney Medical School, The University of 10 Sydney, Sydney, NSW 2006, Australia; [email protected] (WSC); 11 [email protected] (MS); [email protected] (ECH); 12 [email protected] (JP); [email protected] (NL) 13 2WHO Collaborating Centre for Reference and Research on Influenza, at The Peter Doherty 14 Institute for Infection and Immunity, Melbourne, VIC 3000, Australia; 15 [email protected] (MW) 16 3Westmead Institute for Medical Research, Centre for Virus Research, Westmead NSW, 17 2145; Australia; [email protected] (JSE); 18 19 20 * Correspondence: [email protected]; Tel.: +61 2 9351 5591 21 bioRxiv preprint doi: https://doi.org/10.1101/539924; this version posted February 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Hepatitis E Virus Genotype 3 in Shellfish, United Kingdom
LETTERS term to dramatically reduce the high 3. Biswas PK, Christensen JP, Ahmed SS, biologically relevant sentinels and incidence of HPAI in Bangladesh. We Barua H, Das A, Rahman MH. at al. Avian can indicate potential pathogens that infl uenza outbreaks in chickens, Bangla- have progressively and dramatically desh. Emerg Infect Dis. 2008;14:1909–12. are contaminating the environment. increased the scope and benefi ts of our http://dx.doi.org/10.3201/eid1412.071567 It is essential to ensure that this pilot PVC implementation program, 4. Biswas PK, Christensen JP, Ahmed SS, sustainable resource of coastal areas, but additional work is needed. To Das A, Rahman MH, Barua H, et al. where mussels and oysters are farmed Risk for infection with highly pathogenic help spread PVC approaches through- avian infl uenza virus (H5N1) in back- or collected wild, is not subjected to out the country, community leaders, yard chickens, Bangladesh. Emerg In- environmental contamination that imams of local mosques, and school fect Dis. 2009;15:1931–6. http://dx.doi. could lead to public health risks. teachers can be trained to implement org/10.3201/eid1512.090643 Risk management for bivalve 5. Biswas PK, Rahman MH, Das A, Ahmed awareness programs on safe practices SS, Giasuddin M, Christensen JP. Risk mollusks, aimed at control of fecal for raising poultry and regular clean- for highly pathogenic avian infl uenza pollution, relies heavily on the use ing and disinfection of live bird mar- H5N1 virus infection in chickens in of Escherichia coli as an indicator kets. The strengthening of biosecurity small-scale commercial farms, in a high- of fecal (sewage) contamination risk area, Bangladesh, 2008. -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful. -

Emerging Water-Borne Pathogens
Appl Microbiol Biotechnol (2003) 61:424–428 DOI 10.1007/s00253-003-1302-y MINI-REVIEW S. Sharma · P. Sachdeva · J. S. Virdi Emerging water-borne pathogens Received: 1 October 2002 / Revised: 27 February 2003 / Accepted: 28 February 2003 / Published online: 9 April 2003 Springer-Verlag 2003 Abstract Emerging water-borne pathogens constitute a number of reviews and other publications available on the major health hazard in both developed and developing subject discuss emerging and re-emerging pathogens in nations. A new dimension to the global epidemiology of general, including water-borne, without addressing the cholera—an ancient scourge—was provided by the issues specifically pertinent to emerging water-borne emergence of Vibrio cholerae O139. Also, water-borne pathogens. This review critically examines which organ- enterohaemorrhagic Escherichia coli (E. coli O157:H7), isms really qualify as emerging water-borne pathogens, although regarded as a problem of the industrialized west, the possible reasons underlying their emergence and has recently caused outbreaks in Africa. Outbreaks of specific measures to face the challenge posed by them. chlorine-resistant Cryptosporidium have motivated water Due to the paucity of data, it may be difficult to decide authorities to reassess the adequacy of current water- which of all water-borne pathogens are emerging. Nev- quality regulations. Of late, a host of other organisms, ertheless, there are some clear-cut candidates. such as hepatitis viruses (including hepatitis E virus), No organism other than Vibrio cholerae could serve as Campylobacter jejuni, microsporidia, cyclospora, Yersin- a better example of an emerging water-borne pathogen. ia enterocolitica, calciviruses and environmental bacteria Cholera is an ancient scourge and to date seven like Mycobacterium spp, aeromonads, Legionella pneu- pandemics have been recorded. -

Wastewater Based Epidemiology Perspective As a Faster Protocol for Detecting Coronavirus RNA in Human Populations: a Review with Specific Reference to SARS-Cov-2 Virus
pathogens Review Wastewater Based Epidemiology Perspective as a Faster Protocol for Detecting Coronavirus RNA in Human Populations: A Review with Specific Reference to SARS-CoV-2 Virus Milad Mousazadeh 1,2,†,‡ , Razieh Ashoori 3, Biswaranjan Paital 4 , I¸sıkKabda¸slı 5,‡ , Zacharias Frontistis 6 , Marjan Hashemi 7 , Miguel A. Sandoval 8,9,‡ , Samendra Sherchan 10, Kabita Das 11 and Mohammad Mahdi Emamjomeh 12,* 1 Student Research Committee, Qazvin University of Medical Sciences, Qazvin, Iran; [email protected] 2 Department of Environmental Health Engineering, School of Health, Qazvin University of Medical Sciences, Qazvin, Iran 3 Department of Environmental Health Engineering, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran; [email protected] 4 Redox Regulation Laboratory, College of Basic Science and Humanities, Odisha University of Agriculture and Technology, Bhubaneswar 751003, India; [email protected] 5 Environmental Engineering Department, Civil Engineering Faculty, Ayaza˘gaCampus, Istanbul˙ Technical University, Istanbul˙ 34469, Turkey; [email protected] 6 Department of Chemical Engineering, University of Western Macedonia, 50132 Kozani, Greece; Citation: Mousazadeh, M.; Ashoori, [email protected] 7 Environmental and Occupational Hazards Control Research Center, Shahid Beheshti University of Medical R.; Paital, B.; Kabda¸slı,I.; Frontistis, Sciences, Tehran, Iran; [email protected] Z.; Hashemi, M.; Sandoval, M.A.; 8 Laboratorio de Electroquímica Medio Ambiental LEQMA, Departamento -

Preventing Polio WHO Myanmar Newsletter Special , 5 August 2019
Preventing polio WHO Myanmar newsletter special , 5 August 2019 What is polio? Poliomyelitis (polio) is a highly infectious disease that is caused when the polio virus invades the nervous system of an infected person. Polio can cause paralysis and even death. Who is most at risk for polio? Poliovirus usually affects children under 5 years of age who are unvaccinated or under-vaccinated. The virus can also affect or be carried by adolescents and adults. photo credit: Ms Lei Lei Mon, WHO Myanmar The child or individual suspected of polio may A child receives oral polio vaccine at Thandaung township, complain of sudden onset of weakness of arms Kayin State, 21 July 2019 and/or legs. Oral polio vaccine is the vaccine Is there a cure for polio? recommended for polio eradication Can it be prevented? There is no cure for polio. The disease can severely paralyze, or even kill, an infected child. Is the polio vaccine safe? Oral polio vaccine is one of the safest vaccines Polio can be prevented by immunizing a child with ever developed. It is so safe it can be given to sick approrpiate vaccination. There are currently two children and newborns. Since 1961, when oral effective polio vaccines, the inactivated poliovirus polio vaccine was introduced, two billion children vaccine (IPV) and the live attenuated oral polio have been immunized against polio globally. vaccine (OPV). This is estimated to have saved at least 16 million children from permanent paralysis by polio, world- wide. It is also safe to administer multiple doses of polio vaccine to children. -

Disease Reporting-Clinician
Infections, diseases and conditions reportable by clinicians: 2015 Report immediately • Anthrax (Bacillus anthracis) • Botulism (Clostridium botulinum) • Cholera (Vibrio cholerae O1, O139, or toxigenic) • Diphtheria (Corynebacterium diphtheriae) • Hemorrhagic fever caused by viruses of the filovirus (e.g., Ebola, Marburg) or arenavirus (e.g., Lassa, Machupo) families • Influenza (novel)1 (From footnote: Influenza A virus that cannot be subtyped by commercially distributed assays) • Marine intoxication (intoxication caused by marine microorganisms or their byproducts (e.g., paralytic shellfish poisoning, domoic acid intoxication, ciguatera, scombroid) • Measles (rubeola) • Plague (Yersinia pestis) • Poliomyelitis • Rabies (human) • Rubella • SARS (Severe Acute Respiratory Syndrome or SARS-coronavirus) • Smallpox (variola) • Tularemia (Francisella tularensis) • Yellow fever • Outbreaks and uncommon illnesses (any known or suspected common-source outbreak; any uncommon illness of potential public health significance) Report within 24 hours (including weekends and holidays) • Haemophilus influenzae (any isolation or identification from a normally sterile specimen type) • Neisseria meningitidis • Pesticide poisoning Public Health Division | Infections, diseases and conditions reportable by clinicians 128 Report within one working day • Amebic infections (central nervous system only) (for example infection by Naegleria or Balamuthia spp. • Animal bites (of humans) • Arthropod vector-borne disease (babesiosis, California encephalitis, Colorado