W15: What Should You Know About Male Urinary Incontinence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urological Trauma
Guidelines on Urological Trauma D. Lynch, L. Martinez-Piñeiro, E. Plas, E. Serafetinidis, L. Turkeri, R. Santucci, M. Hohenfellner © European Association of Urology 2007 TABLE OF CONTENTS PAGE 1. RENAL TRAUMA 5 1.1 Background 5 1.2 Mode of injury 5 1.2.1 Injury classification 5 1.3 Diagnosis: initial emergency assessment 6 1.3.1 History and physical examination 6 1.3.1.1 Guidelines on history and physical examination 7 1.3.2 Laboratory evaluation 7 1.3.2.1 Guidelines on laboratory evaluation 7 1.3.3 Imaging: criteria for radiographic assessment in adults 7 1.3.3.1 Ultrasonography 7 1.3.3.2 Standard intravenous pyelography (IVP) 8 1.3.3.3 One shot intraoperative intravenous pyelography (IVP) 8 1.3.3.4 Computed tomography (CT) 8 1.3.3.5 Magnetic resonance imaging (MRI) 9 1.3.3.6 Angiography 9 1.3.3.7 Radionuclide scans 9 1.3.3.8 Guidelines on radiographic assessment 9 1.4 Treatment 10 1.4.1 Indications for renal exploration 10 1.4.2 Operative findings and reconstruction 10 1.4.3 Non-operative management of renal injuries 11 1.4.4 Guidelines on management of renal trauma 11 1.4.5 Post-operative care and follow-up 11 1.4.5.1 Guidelines on post-operative management and follow-up 12 1.4.6 Complications 12 1.4.6.1 Guidelines on management of complications 12 1.4.7 Paediatric renal trauma 12 1.4.7.1 Guidelines on management of paediatric trauma 13 1.4.8 Renal injury in the polytrauma patient 13 1.4.8.1 Guidelines on management of polytrauma with associated renal injury 14 1.5 Suggestions for future research studies 14 1.6 Algorithms 14 1.7 References 17 2. -

Urology Services in the ASC
Urology Services in the ASC Brad D. Lerner, MD, FACS, CASC Medical Director Summit ASC President of Chesapeake Urology Associates Chief of Urology Union Memorial Hospital Urologic Consultant NFL Baltimore Ravens Learning Objectives: Describe the numerous basic and advanced urology cases/lines of service that can be provided in an ASC setting Discuss various opportunities regarding clinical, operational and financial aspects of urology lines of service in an ASC setting Why Offer Urology Services in Your ASC? Majority of urologic surgical services are already outpatient Many urologic procedures are high volume, short duration and low cost Increasing emphasis on movement of site of service for surgical cases from hospitals and insurance carriers to ASCs There are still some case types where patients are traditionally admitted or placed in extended recovery status that can be converted to strictly outpatient status and would be suitable for an ASC Potential core of fee-for-service case types (microsurgery, aesthetics, prosthetics, etc.) Increasing Population of Those Aged 65 and Over As of 2018, it was estimated that there were 51 million persons aged 65 and over (15.63% of total population) By 2030, it is expected that there will be 72.1 million persons aged 65 and over National ASC Statistics - 2017 Urology cases represented 6% of total case mix for ASCs Urology cases were 4th in median net revenue per case (approximately $2,400) – behind Orthopedics, ENT and Podiatry Urology comprised 3% of single specialty ASCs (5th behind -

Delineation of Privileges Urology Privileges Provider Name
Delineation Of Privileges Urology Privileges Provider Name: Privilege Requested Deferred Approved UROLOGY PRIVILEGES Criteria - New Applicants:: Board Certification or qualified for certification by the American Board of Urology. Criteria - Current Staff Members Only: Successful completion of an ACGME or AOA approved training program; OR demonstrated acceptable practice in the privileges being requested for a minimum of five (5) years. Proctoring Requirements: A minimum of eight (8) cases, in accordance with the Medical Staff Proctoring Protocol. GENERAL PRIVILEGES: Admit ___ ___ ___ Consultation Only Privileges ___ ___ ___ Surgical Assist Only ___ ___ ___ Local block anesthesia ___ ___ ___ Regional block anesthesia ___ ___ ___ Sedation analgesia ___ ___ ___ Criteria: Requires successful completion of the Sedation Assessment test. Additional criteria effective April 1, 2015: a) Evidence of current ACLS and/or PALS certification from the American Heart Association; AND b) Evidence of completion of an Airway Management Course a) Adult Sedation ___ ___ ___ b) Pediatric Sedation (17 years and under) ___ ___ ___ CATEGORY 1 - UROLOGY PRIVILEGES ___ ___ ___ Includes the management and coordination of care, treatment and services, including: medical history and physical evaluations, consultations and prescribing medication in accordance with DEA certificate. Urethral, bladder catheterization ___ ___ ___ Suprapubic, bladder aspiration ___ ___ ___ Page 1 Printed on Wednesday, December 10, 2014 Delineation Of Privileges Urology Privileges Provider -

A Systematic Review of Graft Augmentation Urethroplasty Techniques for the Treatment of Anterior Urethral Strictures
EUROPEAN UROLOGY 59 (2011) 797–814 available at www.sciencedirect.com journal homepage: www.europeanurology.com Review – Reconstructive Urology A Systematic Review of Graft Augmentation Urethroplasty Techniques for the Treatment of Anterior Urethral Strictures Altaf Mangera *, Jacob M. Patterson, Christopher R. Chapple Royal Hallamshire Hospital, Sheffield, United Kingdom Article info Abstract Article history: Context: Reconstructive surgeons who perform urethroplasty have a variety of Accepted February 2, 2011 techniques in their armamentarium that may be used according to factors such as Published online ahead of aetiology, stricture position, and length. No one technique is recommended. print on February 11, 2011 Objective: Our aim was to assess the reported outcomes of the various techniques for graft augmentation urethroplasty according to site of surgery. Keywords: Evidence acquisition: We performed an updated systematic review of the Medline Augmentation urethroplasty literature from 1985 to date and classified the data according to the site of surgery Anterior urethral stricture and technique used. Data are also presented on the type of graft used and the Bulbar urethroplasty follow-up methodology used by each centre. Dorsal onlay bulbar Evidence synthesis: More than 2000 anterior urethroplasty procedures have been urethroplasty describedinthe literature.Whenconsidering the bulbar urethra there isnosignificant Ventral onlay bulbar difference between the average success rates of the dorsal and the ventral onlay urethroplasty procedures, 88.4% and 88.8% at42.2 and 34.4 moin 934 and 563 patients,respectively. Penile urethroplasty The lateral onlay technique has only been described in six patients and has a reported success rate of 83% at 77 mo. The Asopa and Palminteri techniques have been described in 89 and 53 patients with a success rate of 86.7% and 90.1% at 28.9 and 21.9 mo, respectively. -

(Part 1): Management of Male Urethral Stricture Disease
EURURO-9412; No. of Pages 11 E U R O P E A N U R O L O G Y X X X ( 2 0 2 1 ) X X X – X X X ava ilable at www.sciencedirect.com journa l homepage: www.europeanurology.com Review – Reconstructive Urology European Association of Urology Guidelines on Urethral Stricture Disease (Part 1): Management of Male Urethral Stricture Disease a, b c d Nicolaas Lumen *, Felix Campos-Juanatey , Tamsin Greenwell , Francisco E. Martins , e f a c g Nadir I. Osman , Silke Riechardt , Marjan Waterloos , Rachel Barratt , Garson Chan , h i a j Francesco Esperto , Achilles Ploumidis , Wesley Verla , Konstantinos Dimitropoulos a b Division of Urology, Gent University Hospital, Gent, Belgium; Urology Department, Marques de Valdecilla University Hospital, Santander, Spain; c d Department of Urology, University College London Hospital, London, UK; Department of Urology, Santa Maria University Hospital, University of Lisbon, e f Lisbon, Portugal; Department of Urology, Sheffield Teaching Hospitals, Sheffield, UK; Department of Urology, University Medical Center Hamburg- g h Eppendorf, Hamburg, Germany; Division of Urology, University of Saskatchewan, Saskatoon, Canada; Department of Urology, Campus Biomedico i j University of Rome, Rome, Italy; Department of Urology, Athens Medical Centre, Athens, Greece; Aberdeen Royal Infirmary, Aberdeen, UK Article info Abstract Article history: Objective: To present a summary of the 2021 version of the European Association of Urology (EAU) guidelines on management of male urethral stricture disease. Accepted May 15, 2021 Evidence acquisition: The panel performed a literature review on these topics covering a time frame between 2008 and 2018, and used predefined inclusion and exclusion criteria Associate Editor: for the literature to be selected. -

Outcomes of Urethroplasty to Treat Urethral Strictures Arising from Artificial Urinary Sphincter Erosions and Rates of Subsequent Device Replacement
UCSF UC San Francisco Previously Published Works Title Outcomes of Urethroplasty to Treat Urethral Strictures Arising From Artificial Urinary Sphincter Erosions and Rates of Subsequent Device Replacement. Permalink https://escholarship.org/uc/item/4sk1p5g1 Authors Keihani, Sorena Chandrapal, Jason C Peterson, Andrew C et al. Publication Date 2017-09-01 DOI 10.1016/j.urology.2017.05.049 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Reconstructive Urology Outcomes of Urethroplasty to Treat Urethral Strictures Arising From Artificial Urinary Sphincter Erosions and Rates of Subsequent Device Replacement Sorena Keihani, Jason C. Chandrapal, Andrew C. Peterson, Joshua A. Broghammer, Nathan Chertack, Sean P. Elliott, Keith F. Rourke, Nejd F. Alsikafi, Jill C. Buckley, Benjamin N. Breyer, Thomas G. Smith III, Bryan B. Voelzke, Lee C. Zhao, William O. Brant, and Jeremy B. Myers, for the Trauma and Urologic Reconstruction Network of Surgeons (TURNS, TURNSresearch.org) OBJECTIVE To evaluate the success of urethroplasty for urethral strictures arising after erosion of an artificial urinary sphincter (AUS) and rates of subsequent AUS replacement. PATIENTS AND From 2009-2016, we identified patients from the Trauma and Urologic Reconstruction Network METHODS of Surgeons and several other centers. We included patients with urethral strictures arising from AUS erosion undergoing urethroplasty with or without subsequent AUS replacement. We ret- rospectively reviewed patient demographics, history, stricture characteristics, and outcomes. Vari- ables in patients with and without complications after AUS replacement were compared using chi-square test, independent samples t test, and Mann-Whitney U test when appropriate. RESULTS Thirty-one men were identified with the inclusion criteria. -

Urotoday International Journal Volume 5 - August 2012 Table of Contents: August, 2012
® UIJ UroToday International Journal www.urotodayinternationaljournal.com Volume 5 - August 2012 Table of Contents: August, 2012 Review • Retroperitoneum Beyond the Kidney: Retroperitoneal Sarcomas in a Urology Department BJ Pereira, R Borges, R Leão, V Grenha, H Coelho, A Brandão, P Temido Erectile Dysfunction • Penile Fractures: Tertiary Center Experience and a Review of the Literature Avinash T Siddaiah, Madhumohan R Prabhudessai, Prashant R Lawande, Nilesh B Talwadker, Rajeev N. Palvia, Elphiston Fernandes, Pravin Govardhane, Pankaj D Chari, Prashant TN Mandrekar Endourology • Memokath 044 Stent for the Treatment of Recurrent Bulbar Urethral Strictures Magdy Ahmad Alsayed El-Tabey Reconstruction • Pyeloplasty in Ureteropelvic Junction Obstruction: Laparoscopic or Open? Siavash Falahatkar, Ali Roushani, Hamidreza Nasseh, Ehsan Kazemnezhad, Keivan Gholamjani Moghaddam, Seyedeh Mahboobe Raoofi, Mani Mohiti Asl, Ahmad Enshaei, Alireza Farzan Stone Disease • A Multi-institutional Study Demonstrating the Safety and Efficacy of Holmium Laser Ureterolithotripsy Ahmed S Safwat, Nabil K Bissada, Udaya Kumar, Mahmoud M Osman, Toulupe Bakare, Ehab Eltahawy, Samy Heshmat Case Reports • A Case Report of Foot Drop Following Visual Internal Uretherotomy In the Lithotomy Position: Is Prolonged Surgery In the Lithotomy Position the Only Important Risk Factor? NH Nagaraja, Satyam Sharma, GB Manjuprasad • A Rare Case of Fungus Balls of the Urinary Bladder Due to Candida Tropicalis Sivaneswaran Lechmiannandan, Eng Hong Goh, Boon Wei Teoh, Kah Ann Git -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

CLEVELAND AMBULATORY SURGERY CENTER DELINEATION of CLINICAL PRIVILEGES Urology Applicant’S Signature Date
CLEVELAND AMBULATORY SURGERY CENTER DELINEATION OF CLINICAL PRIVILEGES Urology Applicant’s Signature Date The granting, reviewing and changing of clinical privileges will be in accordance with the Medical Staff Bylaws. Assignment of such clinical privileges must be based upon education, clinical training, demonstrated skills and capacity to manage procedurally related complications. Indicate procedures for which you do and do not wish to be credentialed. Return this form with your Application. Recommendation by Procedures Credentialing Request QM Committee Yes No Yes No Hernia hydrocele repair Repair inguinal hernia w/orchiectomy Repair inguinal hernia w/excision of hydrocele Repair inguinal hernia, recurrent Repair inguinal hernia, sliding Repair ventral hernia Repair ventral hernia, recurrent Repair unbilical hernia, age 5 or over Drain perineal abscess Cath or stent ureter Injection for pyelography thru cath Injection procedure for pyelography Change nephrostomy tube Renal endoscopy thru established nephrostomy Renal endoscopy w/fulguration and/or excision Injection ureterography thru cath Injection vis ileal conduit Ureteral endoscopy w/ureteral cath Ureteral endoscopy w/biopsy Fulguration prostate Urethrotomy pendulous urethra Urethrotomy perineal urethra Meatotomy Meatotomy infant Drainage deep periurethral abscess Excision or fulguration carcinoma urethra Excision urethral diverticulum female Excision urethral diverticulum male Marsup urethral caruncle Excision urethral caruncle Excision urethral prolapse Page 1 of 5 MS2O DELINEATION -

Medicare Quarterly Provider Compliance Newsletter Guidance to Address Billing Errors Volume 3 Issue 4 July 2013
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services Medicare Quarterly Provider Compliance Newsletter Guidance to Address Billing Errors Volume 7, Issue 1 ICN 909346/ October 2016 Comprehensive Error Rate Testing (CERT): Transluminal Balloon Angioplasty, Venous Provider Types Affected: Physicians and Providers Background Percutaneous Transluminal Angioplasty (PTA) is a procedure that can open up a blocked blood vessel using a small, flexible plastic tube, or catheter, with a "balloon" at the end of it. When the tube is in place, it inflates to open the blood vessel so that normal blood flow is restored. This procedure is commonly performed to preserve the function of hemodialysis fistulas. Hemodialysis fistulas are surgically created communications between the native artery and vein in an extremity and provide vascular access in patients on hemodialysis. One of the most challenging aspects of the long-term treatment of dialysis patients is the preservation of patent, well-functioning dialysis fistulas. Percutaneous techniques such as balloon angioplasty allow for the treatment of stenosis and fistula thrombosis without surgical thrombectomy and revision. Description of Special Study The CERT Review Contractor conducted a special study of claims with lines for venous transluminal balloon angioplasty procedures billed with Healthcare Common Procedure Coding System (HCPCS) code 35476 submitted from April through June 2015. When CERT reviews a claim, all lines submitted on the claim undergo complex medical review. The long description of this HCPCS code is: transluminal balloon angioplasty, percutaneous; venous. The consumer-friendly version of this HCPCS code descriptor is: balloon dilation of narrowed or blocked vein, accessed through the skin. -

Direct Visual Internal Urethrotomy After Failed Bulbar Urethroplasty
Surgica l & l U a r ic o l d o e g y M Warner et al., Med Surg Urol 2015, 4:3 Medical & Surgical Urology DOI: 10.4172/2168-9857.1000154 ISSN: 2168-9857 Research Article Open Access Direct Visual Internal Urethrotomy after Failed Bulbar Urethroplasty Jonathan N Warner1, Min S Jun2, Ibraheem M Malkawi2* and Richard A Santucci2 1Department of Urology, University of Michigan, Ann Arbor, Michigan, USA 2Department of Urology, Detroit Medical Center, Detroit, Michigan, USA Abstract Introduction: Ideal management of failed bulbar urethroplasty is ill defined. The role of direct visual internal urethrotomy (DVIU) as salvage treatment for failed urethroplasty warrants exploration. Material and methods: Patients who failed urethroplasty (buccal or anastomotic) for bulbar urethral stricture from April 2000-April 2013 were reviewed. Demographics, prior treatments, stricture length, and operative approach were evaluated for risk of salvage DVIU failure. Results: Forty-five patients underwent 11 anastomotic and 34 buccal mucosal urethroplasties. Mean stricture length was 3.9cm (0.5- 9cm), mean follow-up was 40 months (12-160months) and mean time to failure was 20 months (1-133months). Upon failure, 4 patients underwent redo urethroplasty, and 41 underwent DVIU. Patients treated with immediate redo urethroplasty had no recurrence. 56% (23/41) treated with initial DVIU had no recurrence. Analysis of age, original and recurrent stricture length, and number of preoperative dilations or DVIUs showed no difference between those with and without recurrent stricture after DVIU. No significant difference in failure of salvage DVIU between anastomotic and buccal mucosal grafting was seen. Cox regression analysis showed no significant difference in time to recurrence after salvage DVIU based on anastomotic versus buccal (p=0.3), initial or recurrent stricture length, total prior dilations or DVIUs, and age. -
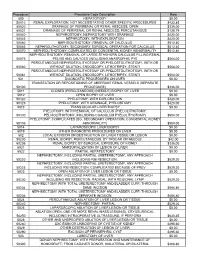
Procedure Procedure Code Description Rate 500
Procedure Procedure Code Description Rate 500 HEPATOTOMY $0.00 50010 RENAL EXPLORATION, NOT NECESSITATING OTHER SPECIFIC PROCEDURES $433.85 50020 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; OPEN $336.00 50021 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; PERCUTANIOUS $128.79 50040 NEPHROSTOMY, NEPHROTOMY WITH DRAINAGE $420.00 50045 NEPHROTOMY, WITH EXPLORATION $420.00 50060 NEPHROLITHOTOMY; REMOVAL OF CALCULUS $512.40 50065 NEPHROLITHOTOMY; SECONDARY SURGICAL OPERATION FOR CALCULUS $512.40 50070 NEPHROLITHOTOMY; COMPLICATED BY CONGENITAL KIDNEY ABNORMALITY $512.40 NEPHROLITHOTOMY; REMOVAL OF LARGE STAGHORN CALCULUS FILLING RENAL 50075 PELVIS AND CALYCES (INCLUDING ANATROPHIC PYE $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50080 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50081 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 501 DIAGNOSTIC PROCEDURES ON LIVER $0.00 TRANSECTION OR REPOSITIONING OF ABERRANT RENAL VESSELS (SEPARATE 50100 PROCEDURE) $336.00 5011 CLOSED (PERCUTANEOUS) (NEEDLE) BIOPSY OF LIVER $0.00 5012 OPEN BIOPSY OF LIVER $0.00 50120 PYELOTOMY; WITH EXPLORATION $420.00 50125 PYELOTOMY; WITH DRAINAGE, PYELOSTOMY $420.00 5013 TRANSJUGULAR LIVER BIOPSY $0.00 PYELOTOMY; WITH REMOVAL OF CALCULUS (PYELOLITHOTOMY, 50130 PELVIOLITHOTOMY, INCLUDING COAGULUM PYELOLITHOTOMY) $504.00 PYELOTOMY; COMPLICATED (EG, SECONDARY OPERATION, CONGENITAL KIDNEY 50135 ABNORMALITY) $504.00 5014 LAPAROSCOPIC LIVER BIOPSY $0.00 5019 OTHER DIAGNOSTIC PROCEDURES