Glossary of Terms Relating to Thermal and Thermo Mechanical Properties of Polymers (IUPAC Recommendations 2013)*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crystal Structures
Crystal Structures Academic Resource Center Crystallinity: Repeating or periodic array over large atomic distances. 3-D pattern in which each atom is bonded to its nearest neighbors Crystal structure: the manner in which atoms, ions, or molecules are spatially arranged. Unit cell: small repeating entity of the atomic structure. The basic building block of the crystal structure. It defines the entire crystal structure with the atom positions within. Lattice: 3D array of points coinciding with atom positions (center of spheres) Metallic Crystal Structures FCC (face centered cubic): Atoms are arranged at the corners and center of each cube face of the cell. FCC continued Close packed Plane: On each face of the cube Atoms are assumed to touch along face diagonals. 4 atoms in one unit cell. a 2R 2 BCC: Body Centered Cubic • Atoms are arranged at the corners of the cube with another atom at the cube center. BCC continued • Close Packed Plane cuts the unit cube in half diagonally • 2 atoms in one unit cell 4R a 3 Hexagonal Close Packed (HCP) • Cell of an HCP lattice is visualized as a top and bottom plane of 7 atoms, forming a regular hexagon around a central atom. In between these planes is a half- hexagon of 3 atoms. • There are two lattice parameters in HCP, a and c, representing the basal and height parameters Volume respectively. 6 atoms per unit cell Coordination number – the number of nearest neighbor atoms or ions surrounding an atom or ion. For FCC and HCP systems, the coordination number is 12. For BCC it’s 8. -

Lab: Specific Heat
Lab: Specific Heat Summary Students relate thermal energy to heat capacity by comparing the heat capacities of different materials and graphing the change in temperature over time for a specific material. Students explore the idea of insulators and conductors in an attempt to apply their knowledge in real world applications, such as those that engineers would. Engineering Connection Engineers must understand the heat capacity of insulators and conductors if they wish to stay competitive in “green” enterprises. Engineers who design passive solar heating systems take advantage of the natural heat capacity characteristics of materials. In areas of cooler climates engineers chose building materials with a low heat capacity such as slate roofs in an attempt to heat the home during the day. In areas of warmer climates tile roofs are popular for their ability to store the sun's heat energy for an extended time and release it slowly after the sun sets, to prevent rapid temperature fluctuations. Engineers also consider heat capacity and thermal energy when they design food containers and household appliances. Just think about your aluminum soda can versus glass bottles! Grade Level: 9-12 Group Size: 4 Time Required: 90 minutes Activity Dependency :None Expendable Cost Per Group : US$ 5 The cost is less if thermometers are available for each group. NYS Science Standards: STANDARD 1 Analysis, Inquiry, and Design STANDARD 4 Performance indicator 2.2 Keywords: conductor, energy, energy storage, heat, specific heat capacity, insulator, thermal energy, thermometer, thermocouple Learning Objectives After this activity, students should be able to: Define heat capacity. Explain how heat capacity determines a material's ability to store thermal energy. -

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Crystal Structure of a Material Is Way in Which Atoms, Ions, Molecules Are Spatially Arranged in 3-D Space
Crystalline Structures – The Basics •Crystal structure of a material is way in which atoms, ions, molecules are spatially arranged in 3-D space. •Crystal structure = lattice (unit cell geometry) + basis (atom, ion, or molecule positions placed on lattice points within the unit cell). •A lattice is used in context when describing crystalline structures, means a 3-D array of points in space. Every lattice point must have identical surroundings. •Unit cell: smallest repetitive volume •Each crystal structure is built by stacking which contains the complete lattice unit cells and placing objects (motifs, pattern of a crystal. A unit cell is chosen basis) on the lattice points: to represent the highest level of geometric symmetry of the crystal structure. It’s the basic structural unit or building block of crystal structure. 7 crystal systems in 3-D 14 crystal lattices in 3-D a, b, and c are the lattice constants 1 a, b, g are the interaxial angles Metallic Crystal Structures (the simplest) •Recall, that a) coulombic attraction between delocalized valence electrons and positively charged cores is isotropic (non-directional), b) typically, only one element is present, so all atomic radii are the same, c) nearest neighbor distances tend to be small, and d) electron cloud shields cores from each other. •For these reasons, metallic bonding leads to close packed, dense crystal structures that maximize space filling and coordination number (number of nearest neighbors). •Most elemental metals crystallize in the FCC (face-centered cubic), BCC (body-centered cubic, or HCP (hexagonal close packed) structures: Room temperature crystal structure Crystal structure just before it melts 2 Recall: Simple Cubic (SC) Structure • Rare due to low packing density (only a-Po has this structure) • Close-packed directions are cube edges. -

Determination of the Molar Mass of an Unknown Solid by Freezing Point Depression
Determination of the Molar Mass of an Unknown Solid by Freezing Point Depression GOAL AND OVERVIEW In the first part of the lab, a series of solutions will be made in order to determine the freezing point depression constant, Kf, for cyclohexane. The freezing points of these solutions, which will contain known amounts of p-dichlorobenzene dissolved in cyclohexane, will be measured. In the second part of the lab, the freezing point of a cyclohexane solution will be prepared that contains a known mass of an unknown organic solid. The measured freezing point change will be used to calculate the molar mass of the unknown solid. Objectives and Science Skills • Understand and explain colligative properties of solutions, including freezing point depression. • Measure the freezing point of a pure solvent and of a solution in that solvent containing an unknown solute. • Calculate the molar mass of the unknown solute and determine its probable identity from a set of choices. • Quantify the expected freezing point change in a solution of known molality. • Quantitatively and qualitatively compare experimental results with theoretical values. • Identify and discuss factors or effects that may contribute to the uncertainties in values determined from experimental data. SUGGESTED REVIEW AND EXTERNAL READING • Reference information on thermodynamics; data analysis; relevant textbook information BACKGROUND If a liquid is at a higher temperature than its surroundings, the liquid's temperature will fall as it gives up heat to the surroundings. When the liquid's temperature reaches its freezing point, the liquid's temperature will not fall as it continues to give up heat to the surroundings. -

Compression of an Ideal Gas with Temperature Dependent Specific
Session 3666 Compression of an Ideal Gas with Temperature-Dependent Specific Heat Capacities Donald W. Mueller, Jr., Hosni I. Abu-Mulaweh Engineering Department Indiana University–Purdue University Fort Wayne Fort Wayne, IN 46805-1499, USA Overview The compression of gas in a steady-state, steady-flow (SSSF) compressor is an important topic that is addressed in virtually all engineering thermodynamics courses. A typical situation encountered is when the gas inlet conditions, temperature and pressure, are known and the compressor dis- charge pressure is specified. The traditional instructional approach is to make certain assumptions about the compression process and about the gas itself. For example, a special case often consid- ered is that the compression process is reversible and adiabatic, i.e. isentropic. The gas is usually ÊÌ considered to be ideal, i.e. ÈÚ applies, with either constant or temperature-dependent spe- cific heat capacities. The constant specific heat capacity assumption allows for direct computation of the discharge temperature, while the temperature-dependent specific heat assumption does not. In this paper, a MATLAB computer model of the compression process is presented. The substance being compressed is air which is considered to be an ideal gas with temperature-dependent specific heat capacities. Two approaches are presented to describe the temperature-dependent properties of air. The first approach is to use the ideal gas table data from Ref. [1] with a look-up interpolation scheme. The second approach is to use a curve fit with the NASA Lewis coefficients [2,3]. The computer model developed makes use of a bracketing–bisection algorithm [4] to determine the temperature of the air after compression. -
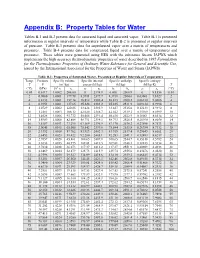
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

Origin of Probability in Quantum Mechanics and the Physical Interpretation of the Wave Function
Origin of Probability in Quantum Mechanics and the Physical Interpretation of the Wave Function Shuming Wen ( [email protected] ) Faculty of Land and Resources Engineering, Kunming University of Science and Technology. Research Article Keywords: probability origin, wave-function collapse, uncertainty principle, quantum tunnelling, double-slit and single-slit experiments Posted Date: November 16th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-95171/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Origin of Probability in Quantum Mechanics and the Physical Interpretation of the Wave Function Shuming Wen Faculty of Land and Resources Engineering, Kunming University of Science and Technology, Kunming 650093 Abstract The theoretical calculation of quantum mechanics has been accurately verified by experiments, but Copenhagen interpretation with probability is still controversial. To find the source of the probability, we revised the definition of the energy quantum and reconstructed the wave function of the physical particle. Here, we found that the energy quantum ê is 6.62606896 ×10-34J instead of hν as proposed by Planck. Additionally, the value of the quality quantum ô is 7.372496 × 10-51 kg. This discontinuity of energy leads to a periodic non-uniform spatial distribution of the particles that transmit energy. A quantum objective system (QOS) consists of many physical particles whose wave function is the superposition of the wave functions of all physical particles. The probability of quantum mechanics originates from the distribution rate of the particles in the QOS per unit volume at time t and near position r. Based on the revision of the energy quantum assumption and the origin of the probability, we proposed new certainty and uncertainty relationships, explained the physical mechanism of wave-function collapse and the quantum tunnelling effect, derived the quantum theoretical expression of double-slit and single-slit experiments. -

Chapter 10 – Chemical Reactions Notes
Chapter 8 – Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if a chemical reaction has occurred (recap): Temperature changes that can’t be accounted for. o Exothermic reactions give off energy (as in fire). o Endothermic reactions absorb energy (as in a cold pack). Spontaneous color change. o This happens when things rust, when they rot, and when they burn. Appearance of a solid when two liquids are mixed. o This solid is called a precipitate. Formation of a gas / bubbling, as when vinegar and baking soda are mixed. Overall, the most important thing to remember is that a chemical reaction produces a whole new chemical compound. Just changing the way that something looks (breaking, melting, dissolving, etc) isn’t enough to qualify something as a chemical reaction! Balancing Equations Notes: Things to keep in mind when looking at the recipes for chemical reactions: 1) The stuff before the arrow is referred to as the “reactants” or “reagents”, and the stuff after the arrow is called the “products.” 2) The number of atoms of each element is the same on both sides of the arrow. Even though there may be different numbers of molecules, the number of atoms of each element needs to remain the same to obey the law of conservation of mass. 3) The numbers in front of the formulas tell you how many molecules or moles of each chemical are involved in the reaction. 4) Equations are nothing more than chemical recipes. -

Sounds of a Supersolid A
NEWS & VIEWS RESEARCH hypothesis came from extensive population humans, implying possible mosquito exposure long-distance spread of insecticide-resistant time-series analysis from that earlier study5, to malaria parasites and the potential to spread mosquitoes, worsening an already dire situ- which showed beyond reasonable doubt that infection over great distances. ation, given the current spread of insecticide a mosquito vector species called Anopheles However, the authors failed to detect resistance in mosquito populations. This would coluzzii persists locally in the dry season in parasite infections in their aerially sampled be a matter of great concern because insecticides as-yet-undiscovered places. However, the malaria vectors, a result that they assert is to be are the best means of malaria control currently data were not consistent with this outcome for expected given the small sample size and the low available8. However, long-distance migration other malaria vectors in the study area — the parasite-infection rates typical of populations of could facilitate the desirable spread of mosqui- species Anopheles gambiae and Anopheles ara- malaria vectors. A problem with this argument toes for gene-based methods of malaria-vector biensis — leaving wind-powered long-distance is that the typical infection rates they mention control. One thing is certain, Huestis and col- migration as the only remaining possibility to are based on one specific mosquito body part leagues have permanently transformed our explain the data5. (salivary glands), rather than the unknown but understanding of African malaria vectors and Both modelling6 and genetic studies7 undoubtedly much higher infection rates that what it will take to conquer malaria. -

Chemistry - B.S
Chemistry - B.S. College of (Biochemistry Option) Arts and Sciences The Department of Chemistry offers the Bachelor of Science degree for students who Graduation Composition and Communication Requirement intend to become professional chemists or do graduate work in chemistry or a closely (GCCR) related discipline. There are three options in the B.S. program: a traditional track WRD 310 Writing in the Natural Sciences ............................................................. 3 covering all the major areas of chemistry, an option that emphasizes biochemistry and an option in materials chemistry. The Biochemistry and Traditional Options are Graduation Composition and Communication certified by the American Chemical Society. A Bachelor of Arts degree program is Requirement hours (GCCR) .................................................................... 3 offered as well for students who want greater flexibility in the selection of courses to perhaps pursue more diverse degree options, including dual and double majors. For College Requirements all majors CHE 109 and CHE 110 have been defined as equivalent to CHE 105. The I. Foreign Language (placement exam recommended) ................................... 0-14 Department also offers the Master of Science and the Doctor of Philosophy degree. II. Disciplinary Requirements a. Natural Science (completed by Major Requirements) 128 hours b. Social Science ......................................................................................... 3 Any student earning a Bachelor of Science (BS) -

Engineering Viscoelasticity
ENGINEERING VISCOELASTICITY David Roylance Department of Materials Science and Engineering Massachusetts Institute of Technology Cambridge, MA 02139 October 24, 2001 1 Introduction This document is intended to outline an important aspect of the mechanical response of polymers and polymer-matrix composites: the field of linear viscoelasticity. The topics included here are aimed at providing an instructional introduction to this large and elegant subject, and should not be taken as a thorough or comprehensive treatment. The references appearing either as footnotes to the text or listed separately at the end of the notes should be consulted for more thorough coverage. Viscoelastic response is often used as a probe in polymer science, since it is sensitive to the material’s chemistry and microstructure. The concepts and techniques presented here are important for this purpose, but the principal objective of this document is to demonstrate how linear viscoelasticity can be incorporated into the general theory of mechanics of materials, so that structures containing viscoelastic components can be designed and analyzed. While not all polymers are viscoelastic to any important practical extent, and even fewer are linearly viscoelastic1, this theory provides a usable engineering approximation for many applications in polymer and composites engineering. Even in instances requiring more elaborate treatments, the linear viscoelastic theory is a useful starting point. 2 Molecular Mechanisms When subjected to an applied stress, polymers may deform by either or both of two fundamen- tally different atomistic mechanisms. The lengths and angles of the chemical bonds connecting the atoms may distort, moving the atoms to new positions of greater internal energy.