Lethal Synthesis of Methylglyoxal by Escherichia Coli During Unregulated Glycerol Metabolism1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electron Transport Phosphorylation in Rumen Butyrivibrios: Unprecedented ATP Yield for Glucose Fermentation to Butyrate
HYPOTHESIS AND THEORY published: 24 June 2015 doi: 10.3389/fmicb.2015.00622 Electron transport phosphorylation in rumen butyrivibrios: unprecedented ATP yield for glucose fermentation to butyrate Timothy J. Hackmann1 and Jeffrey L. Firkins2* 1 Department of Animal Sciences, University of Florida, Gainesville, FL, USA, 2 Department of Animal Sciences, The Ohio State University, Columbus, OH, USA From a genomic analysis of rumen butyrivibrios (Butyrivibrio and Pseudobutyrivibrio sp.), we have re-evaluated the contribution of electron transport phosphorylation (ETP) to ATP formation in this group. This group is unique in that most (76%) genomes were predicted to possess genes for both Ech and Rnf transmembrane ion pumps. These pumps act in concert with the NifJ and Bcd-Etf to form a electrochemical potential (μH+ and μNa+), which drives ATP synthesis by ETP. Of the 62 total butyrivibrio genomes currently available from the Hungate 1000 project, all 62 were predicted to Edited by: possess NifJ, which reduces oxidized ferredoxin (Fdox) during pyruvate conversion to Emilio M. Ungerfeld, Instituto de Investigaciones acetyl-CoA. All 62 possessed all subunits of Bcd-Etf, which reduces Fdox and oxidizes Agropecuarias, Chile reduced NAD during crotonyl-CoA reduction. Additionally, 61 genomes possessed all Reviewed by: subunits of the Rnf, which generates μH+ or μNa+ from oxidation of reduced Fd Wolfgang Buckel, (Fdred) and reduction of oxidized NAD. Further, 47 genomes possessed all six subunits Philipps-Universität Marburg, + Germany of the Ech, which generates μH from oxidation of Fdred. For glucose fermentation Robert J. Wallace, to butyrate and H2, the electrochemical potential established should drive synthesis University of Aberdeen, UK of ∼1.5 ATP by the F0F1-ATP synthase (possessed by all 62 genomes). -

Supplementary Informations SI2. Supplementary Table 1
Supplementary Informations SI2. Supplementary Table 1. M9, soil, and rhizosphere media composition. LB in Compound Name Exchange Reaction LB in soil LBin M9 rhizosphere H2O EX_cpd00001_e0 -15 -15 -10 O2 EX_cpd00007_e0 -15 -15 -10 Phosphate EX_cpd00009_e0 -15 -15 -10 CO2 EX_cpd00011_e0 -15 -15 0 Ammonia EX_cpd00013_e0 -7.5 -7.5 -10 L-glutamate EX_cpd00023_e0 0 -0.0283302 0 D-glucose EX_cpd00027_e0 -0.61972444 -0.04098397 0 Mn2 EX_cpd00030_e0 -15 -15 -10 Glycine EX_cpd00033_e0 -0.0068175 -0.00693094 0 Zn2 EX_cpd00034_e0 -15 -15 -10 L-alanine EX_cpd00035_e0 -0.02780553 -0.00823049 0 Succinate EX_cpd00036_e0 -0.0056245 -0.12240603 0 L-lysine EX_cpd00039_e0 0 -10 0 L-aspartate EX_cpd00041_e0 0 -0.03205557 0 Sulfate EX_cpd00048_e0 -15 -15 -10 L-arginine EX_cpd00051_e0 -0.0068175 -0.00948672 0 L-serine EX_cpd00054_e0 0 -0.01004986 0 Cu2+ EX_cpd00058_e0 -15 -15 -10 Ca2+ EX_cpd00063_e0 -15 -100 -10 L-ornithine EX_cpd00064_e0 -0.0068175 -0.00831712 0 H+ EX_cpd00067_e0 -15 -15 -10 L-tyrosine EX_cpd00069_e0 -0.0068175 -0.00233919 0 Sucrose EX_cpd00076_e0 0 -0.02049199 0 L-cysteine EX_cpd00084_e0 -0.0068175 0 0 Cl- EX_cpd00099_e0 -15 -15 -10 Glycerol EX_cpd00100_e0 0 0 -10 Biotin EX_cpd00104_e0 -15 -15 0 D-ribose EX_cpd00105_e0 -0.01862144 0 0 L-leucine EX_cpd00107_e0 -0.03596182 -0.00303228 0 D-galactose EX_cpd00108_e0 -0.25290619 -0.18317325 0 L-histidine EX_cpd00119_e0 -0.0068175 -0.00506825 0 L-proline EX_cpd00129_e0 -0.01102953 0 0 L-malate EX_cpd00130_e0 -0.03649016 -0.79413596 0 D-mannose EX_cpd00138_e0 -0.2540567 -0.05436649 0 Co2 EX_cpd00149_e0 -
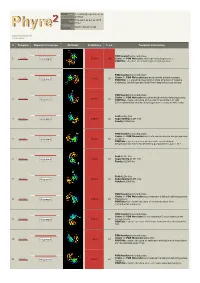
Phyre 2 Results for P25553
Email [email protected] Description P25553 Thu Jan 5 11:42:11 GMT Date 2012 Unique Job 5024f4b9e5342484 ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:oxidoreductase 1 c2hg2A_ Alignment 100.0 100 Chain: A: PDB Molecule:aldehyde dehydrogenase a; PDBTitle: structure of lactaldehyde dehydrogenase PDB header:oxidoreductase Chain: B: PDB Molecule:betaine aldehyde dehydrogenase; 2 c3ed6B_ 100.0 36 Alignment PDBTitle: 1.7 angstrom resolution crystal structure of betaine aldehyde2 dehydrogenase (betb) from staphylococcus aureus PDB header:oxidoreductase Chain: A: PDB Molecule:formyltetrahydrofolate dehydrogenase; 3 c2o2qA_ 100.0 33 Alignment PDBTitle: crystal structure of the c-terminal domain of rat2 10'formyltetrahydrofolate dehydrogenase in complex with nadp Fold:ALDH-like 4 d1a4sa_ Alignment 100.0 35 Superfamily:ALDH-like Family:ALDH-like PDB header:oxidoreductase Chain: H: PDB Molecule:succinate-semialdehyde dehydrogenase 5 c3ifgH_ Alignment 100.0 34 (nadp+); PDBTitle: crystal structure of succinate-semialdehyde dehydrogenase from2 burkholderia pseudomallei, part 1 of 2 Fold:ALDH-like 6 d1bxsa_ Alignment 100.0 33 Superfamily:ALDH-like Family:ALDH-like Fold:ALDH-like 7 d1o9ja_ Alignment 100.0 33 Superfamily:ALDH-like Family:ALDH-like PDB header:oxidoreductase Chain: A: PDB Molecule:succinate-semialdehyde dehydrogenase 8 c3rh9A_ Alignment 100.0 35 (nad(p)(+)); PDBTitle: the crystal structure of oxidoreductase from marinobacter aquaeolei PDB header:oxidoreductase Chain: B: PDB Molecule:5-carboxymethyl-2-hydroxymuconate 9 c2d4eB_ Alignment 100.0 32 semialdehyde PDBTitle: crystal structure of the hpcc from thermus thermophilus hb8 PDB header:oxidoreductase Chain: G: PDB Molecule:antiquitin; 10 c2jg7G_ 100.0 28 Alignment PDBTitle: crystal structure of seabream antiquitin and elucidation of2 its substrate specificity PDB header:oxidoreductase Chain: C: PDB Molecule:succinate-semialdehyde dehydrogenase 11 c3jz4C_ 100.0 39 Alignment [nadp+]; PDBTitle: crystal structure of e. -

Table 4. V. Cholerae Flexgene ORF Collection
Table 4. V. cholerae FLEXGene ORF collection Reference Clone protein PlasmID clone GenBank Locus tag Symbol accession identifier FLEX clone name accession Product name VC0001 NP_062585 VcCD00019918 FLH200476.01F DQ772770 hypothetical protein VC0002 mioC NP_062586 VcCD00019938 FLH200506.01F DQ772771 mioC protein VC0003 thdF NP_062587 VcCD00019958 FLH200531.01F DQ772772 thiophene and furan oxidation protein ThdF VC0004 yidC NP_062588 VcCD00019970 FLH200545.01F DQ772773 inner membrane protein, 60 kDa VC0005 NP_062589 VcCD00061243 FLH236482.01F DQ899316 conserved hypothetical protein VC0006 rnpA NP_062590 VcCD00025697 FLH214799.01F DQ772774 ribonuclease P protein component VC0007 rpmH NP_062591 VcCD00061229 FLH236450.01F DQ899317 ribosomal protein L34 VC0008 NP_062592 VcCD00019917 FLH200475.01F DQ772775 amino acid ABC transporter, ATP-binding protein VC0009 NP_062593 VcCD00019966 FLH200540.01F DQ772776 amino acid ABC transproter, permease protein VC0010 NP_062594 VcCD00019152 FLH199275.01F DQ772777 amino acid ABC transporter, periplasmic amino acid-binding portion VC0011 NP_062595 VcCD00019151 FLH199274.01F DQ772778 hypothetical protein VC0012 dnaA NP_062596 VcCD00017363 FLH174286.01F DQ772779 chromosomal DNA replication initiator DnaA VC0013 dnaN NP_062597 VcCD00017316 FLH174063.01F DQ772780 DNA polymerase III, beta chain VC0014 recF NP_062598 VcCD00019182 FLH199319.01F DQ772781 recF protein VC0015 gyrB NP_062599 VcCD00025458 FLH174642.01F DQ772782 DNA gyrase, subunit B VC0016 NP_229675 VcCD00019198 FLH199346.01F DQ772783 hypothetical protein -

Fermentation of Dihydroxyacetone by Engineered Escherichia Coli and Klebsiella Variicola to Products
Fermentation of dihydroxyacetone by engineered Escherichia coli and Klebsiella variicola to products Liang Wanga, Diane Chauliaca,1, Mun Su Rheea,2, Anushadevi Panneerselvama, Lonnie O. Ingrama,3, and K. T. Shanmugama,3 aDepartment of Microbiology and Cell Science, University of Florida, Gainesville, FL 32611 Contributed by Lonnie O. Ingram, March 21, 2018 (sent for review January 18, 2018; reviewed by John W. Frost and F. Robert Tabita) Methane can be converted to triose dihydroxyacetone (DHA) by process, formaldehyde can also be produced biologically from chemical processes with formaldehyde as an intermediate. Carbon CO2 with formate as an intermediate (Fig. 1) (7). Dickens and dioxide, a by-product of various industries including ethanol/ Williamson reported as early as 1958 that DHA can be produced butanol biorefineries, can also be converted to formaldehyde biologically by transketolation of hydroxypyruvate and formalde- and then to DHA. DHA, upon entry into a cell and phosphorylation hyde (8). This transketolase is implicated in a unique pentose– to DHA-3-phosphate, enters the glycolytic pathway and can be phosphate–dependent pathway (DHA cycle) in methanol-utilizing fermented to any one of several products. However, DHA is yeast that fixes formaldehyde to xylulose-5-phosphate, yielding inhibitory to microbes due to its chemical interaction with cellular DHA as an intermediate in the production of glyceraldehyde-3- components. Fermentation of DHA to D-lactate by Escherichia coli phosphate in a cyclic mode (9). DHA in the cytoplasm is phos- strain TG113 was inefficient, and growth was inhibited by 30 g·L−1 phorylated by DHA kinase and/or glycerol kinase, and the DHA-P DHA. -

Viewed and Published Immediately Upon Acceptance Cited in Pubmed and Archived on Pubmed Central Yours — You Keep the Copyright
BMC Bioinformatics BioMed Central Methodology article Open Access Optimization based automated curation of metabolic reconstructions Vinay Satish Kumar1, Madhukar S Dasika2 and Costas D Maranas*2 Address: 1Department of Industrial and Manufacturing Engineering, The Pennsylvania State University, University Park, PA 16802, USA and 2Department of Chemical Engineering, The Pennsylvania State University, University Park, PA 16802, USA Email: Vinay Satish Kumar - [email protected]; Madhukar S Dasika - [email protected]; Costas D Maranas* - [email protected] * Corresponding author Published: 20 June 2007 Received: 14 December 2006 Accepted: 20 June 2007 BMC Bioinformatics 2007, 8:212 doi:10.1186/1471-2105-8-212 This article is available from: http://www.biomedcentral.com/1471-2105/8/212 © 2007 Satish Kumar et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Currently, there exists tens of different microbial and eukaryotic metabolic reconstructions (e.g., Escherichia coli, Saccharomyces cerevisiae, Bacillus subtilis) with many more under development. All of these reconstructions are inherently incomplete with some functionalities missing due to the lack of experimental and/or homology information. A key challenge in the automated generation of genome-scale reconstructions is the elucidation of these gaps and the subsequent generation of hypotheses to bridge them. Results: In this work, an optimization based procedure is proposed to identify and eliminate network gaps in these reconstructions. First we identify the metabolites in the metabolic network reconstruction which cannot be produced under any uptake conditions and subsequently we identify the reactions from a customized multi-organism database that restores the connectivity of these metabolites to the parent network using four mechanisms. -

Protein Glycation and Methylglyoxal Metabolism in Parkinson's Disease
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE QUÍMICA E BIOQUÍMICA Protein glycation and methylglyoxal metabolism in Parkinson’s disease Hugo Miguel Vicente Miranda Doutoramento em Bioquímica (Especialidade: Regulação Bioquímica) 2009 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE QUÍMICA E BIOQUÍMICA Protein glycation and methylglyoxal metabolism in Parkinson’s disease Hugo Miguel Vicente Miranda Doutoramento em Bioquímica (Especialidade: Regulação Bioquímica) Tese orientada pelo Doutor Carlos Alberto Alves Cordeiro e pelo Prof. Doutor Alexandre Luís de Matos Botica Cortês Quintas 2009 De acordo com o disposto no artigo n°. 40 do Regulamento de Estudos Pós-Graduados da Universidade de Lisboa, Deliberação n° 961/2003, publicada no Diário da República – II Série n°. 153 – 5 de Julho de 2003, foram incluídos nesta dissertação os resultados dos seguintes artigos: Gomes RA, Sousa Silva M, Vicente Miranda H, Ferreira AEN, Cordeiro C, Ponces Freire A. 2005. Protein glycation in Saccharomyces cerevisiae: Argpyrimidine formation and methylglyoxal catabolism. FEBS J. 272: 4521-4531. Gomes RA, Vicente Miranda H, Sousa Silva M, Graça G, Coelho AV, Ferreira AEN, Cordeiro C, Ponces Freire A. 2006. Yeast protein glycation in vivo by methylglyoxal: Molecular modification of glycolytic enzymes and heat shock proteins. FEBS J. 273: 5273-5287. Vicente Miranda H, Ferreira AEN, Cordeiro C, Ponces Freire A. 2006. Kinetic assay for measurement of enzyme concentration in situ. Anal Biochem 354: 148-150. Vicente Miranda H, Ferreira AEN, Quintas A, Cordeiro C, Ponces Freire A. 2008. Measuring intracellular enzyme concentrations. BAMBED 36(2): 135-138. Gomes RA, Vicente Miranda H, Sousa Silva M, Graça G, Coelho AV, Ferreira AEN, Cordeiro C, Ponces Freire A. -

Carbonyl Stress in Bacteria: Causes and Consequences
ISSN 0006-2979, Biochemistry (Moscow), 2015, Vol. 80, No. 13, pp. 1655-1671. © Pleiades Publishing, Ltd., 2015. Original Russian Text © O. V. Kosmachevskaya, K. B. Shumaev, A. F. Topunov, 2015, published in Uspekhi Biologicheskoi Khimii, 2015, Vol. 55, pp. 49-82. REVIEW Carbonyl Stress in Bacteria: Causes and Consequences O. V. Kosmachevskaya, K. B. Shumaev, and A. F. Topunov* Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, 119071 Moscow, Russia; E-mail: [email protected] Received May 30, 2015 Abstract—Pathways of synthesis of the α-reactive carbonyl compound methylglyoxal (MG) in prokaryotes are described in this review. Accumulation of MG leads to development of carbonyl stress. Some pathways of MG formation are similar for both pro- and eukaryotes, but there are reactions specific for prokaryotes, e.g. the methylglyoxal synthase reaction. This reaction and the glyoxalase system constitute an alternative pathway of glucose catabolism – the MG shunt not associated with the synthesis of ATP. In violation of the regulation of metabolism, the cell uses MG shunt as well as other glycolysis shunting pathways and futile cycles enabling stabilization of its energetic status. MG was first examined as a biologically active metabolic factor participating in the formation of phenotypic polymorphism and hyperpersistent potential of bacte- rial populations. The study of carbonyl stress is interesting for evolutionary biology and can be useful for constructing high- ly effective producer strains. DOI: 10.1134/S0006297915130039 Key words: carbonyl stress, bacteria, methylglyoxal, metabolite overproduction The term “carbonyl stress” that describes an imbal- betes, which initiated studies of nonenzymatically glycat- ance between the formation and removal of reactive car- ed proteins and AGEs in living organisms. -

Supplemental Table S1: Comparison of the Deleted Genes in the Genome-Reduced Strains
Supplemental Table S1: Comparison of the deleted genes in the genome-reduced strains Legend 1 Locus tag according to the reference genome sequence of B. subtilis 168 (NC_000964) Genes highlighted in blue have been deleted from the respective strains Genes highlighted in green have been inserted into the indicated strain, they are present in all following strains Regions highlighted in red could not be deleted as a unit Regions highlighted in orange were not deleted in the genome-reduced strains since their deletion resulted in severe growth defects Gene BSU_number 1 Function ∆6 IIG-Bs27-47-24 PG10 PS38 dnaA BSU00010 replication initiation protein dnaN BSU00020 DNA polymerase III (beta subunit), beta clamp yaaA BSU00030 unknown recF BSU00040 repair, recombination remB BSU00050 involved in the activation of biofilm matrix biosynthetic operons gyrB BSU00060 DNA-Gyrase (subunit B) gyrA BSU00070 DNA-Gyrase (subunit A) rrnO-16S- trnO-Ala- trnO-Ile- rrnO-23S- rrnO-5S yaaC BSU00080 unknown guaB BSU00090 IMP dehydrogenase dacA BSU00100 penicillin-binding protein 5*, D-alanyl-D-alanine carboxypeptidase pdxS BSU00110 pyridoxal-5'-phosphate synthase (synthase domain) pdxT BSU00120 pyridoxal-5'-phosphate synthase (glutaminase domain) serS BSU00130 seryl-tRNA-synthetase trnSL-Ser1 dck BSU00140 deoxyadenosin/deoxycytidine kinase dgk BSU00150 deoxyguanosine kinase yaaH BSU00160 general stress protein, survival of ethanol stress, SafA-dependent spore coat yaaI BSU00170 general stress protein, similar to isochorismatase yaaJ BSU00180 tRNA specific adenosine -

12) United States Patent (10
US007635572B2 (12) UnitedO States Patent (10) Patent No.: US 7,635,572 B2 Zhou et al. (45) Date of Patent: Dec. 22, 2009 (54) METHODS FOR CONDUCTING ASSAYS FOR 5,506,121 A 4/1996 Skerra et al. ENZYME ACTIVITY ON PROTEIN 5,510,270 A 4/1996 Fodor et al. MICROARRAYS 5,512,492 A 4/1996 Herron et al. 5,516,635 A 5/1996 Ekins et al. (75) Inventors: Fang X. Zhou, New Haven, CT (US); 5,532,128 A 7/1996 Eggers Barry Schweitzer, Cheshire, CT (US) 5,538,897 A 7/1996 Yates, III et al. s s 5,541,070 A 7/1996 Kauvar (73) Assignee: Life Technologies Corporation, .. S.E. al Carlsbad, CA (US) 5,585,069 A 12/1996 Zanzucchi et al. 5,585,639 A 12/1996 Dorsel et al. (*) Notice: Subject to any disclaimer, the term of this 5,593,838 A 1/1997 Zanzucchi et al. patent is extended or adjusted under 35 5,605,662 A 2f1997 Heller et al. U.S.C. 154(b) by 0 days. 5,620,850 A 4/1997 Bamdad et al. 5,624,711 A 4/1997 Sundberg et al. (21) Appl. No.: 10/865,431 5,627,369 A 5/1997 Vestal et al. 5,629,213 A 5/1997 Kornguth et al. (22) Filed: Jun. 9, 2004 (Continued) (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2005/O118665 A1 Jun. 2, 2005 EP 596421 10, 1993 EP 0619321 12/1994 (51) Int. Cl. EP O664452 7, 1995 CI2O 1/50 (2006.01) EP O818467 1, 1998 (52) U.S. -

Product Sheet Info
Master Clone List for NR-19280 Yersinia pestis Strain KIM Gateway® Clone Set, Recombinant in Escherichia coli, Plates 1-43 Catalog No. NR-19280 Table 1: Yersinia pestis Gateway® Clone, Plate 1 (UYPVA), NR-195971 Clone Well Locus ID Description ORF Accession Average Position Length Number Depth of Coverage 38250 A01 NTL02YP3655 transcriptional activator of nhaA 900 AAM87251.1 3.90531915 38268 A02 NTL02YP0999 apbA protein 912 AAM84595.1 6.01785714 38344 A03 NTL02YP3653 putative regulator 939 AAM87249.1 5.6680286 38375 A04 NTL02YP3668 homoserine kinase 951 AAM87264.1 5.57214934 38386 A05 NTL02YP1006 cytochrome o ubiquinol oxidase subunit II 957 AAM84602.1 5.45937813 38408 A06 NTL02YP2211 suppressor of htrB, heat shock protein 963 AAM85807.1 5.40877368 38447 A07 NTL02YP3649 penicillin tolerance protein 978 AAM87245.1 5.34086444 38477 A08 NTL02YP0988 thiamin-monophosphate kinase 990 AAM84584.1 6.8223301 36045 A09 NTL02YP3670 hypothetical protein 159 AAM87266.1 4.53266332 36094 A10 NTL02YP3672 hypothetical protein 174 AAM87268.1 4.72429907 36136 A11 NTL02YP1000 hypothetical protein 189 AAM84596.1 5.25327511 36241 A12 NTL02YP2577 hypothetical protein 225 AAM86173.1 3.88301887 membrane-bound ATP synthase, F0 36280 B01 NTL02YP4086 240 AAM87682.1 4.79285714 sector, subunit c 36342 B02 NTL02YP3654 30S ribosomal subunit protein S20 264 AAM87250.1 3.86513158 36362 B03 NTL02YP2570 hypothetical protein 270 AAM86166.1 5.40322581 38504 B04 NTL02YP2573 putative ABC transporter permease 996 AAM86169.1 6.65444015 38516 B05 NTL02YP0234 putative heat shock -

A Core of Functionally Complementary Bacteria Colonizes Oysters in Pacific
A core of functionally complementary bacteria colonizes oysters in Pacific Oyster Mortality Syndrome Aude Lucasson, Xing Luo, Shogofa Mortaza, Julien de Lorgeril, Eve Toulza, Bruno Petton, Jean-Michel Escoubas, Camille Clerissi, Lionel Dégremont, Yannick Gueguen, et al. To cite this version: Aude Lucasson, Xing Luo, Shogofa Mortaza, Julien de Lorgeril, Eve Toulza, et al.. A core of func- tionally complementary bacteria colonizes oysters in Pacific Oyster Mortality Syndrome. 2020. hal- 03054359 HAL Id: hal-03054359 https://hal.archives-ouvertes.fr/hal-03054359 Preprint submitted on 11 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. bioRxiv preprint doi: https://doi.org/10.1101/2020.11.16.384644; this version posted November 16, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 A core of functionally complementary bacteria colonizes oysters in Pacific Oyster Mortality 2 Syndrome. 3 4 Aude Lucasson1#, Xing Luo2#, Shogofa Mortaza2#, Julien de Lorgeril1, Eve Toulza1, Bruno Petton3, 5 Jean-Michel Escoubas1, Camille Clerissi4, Lionel Dégremont5, Yannick Gueguen1, Delphine 6 Destoumieux-Garzόn1, Annick Jacq2,& and Guillaume Mitta1,& 7 8 1 IHPE, Université de Montpellier, CNRS, Ifremer, Université de Perpignan Via Domitia, Place E.