Transcriptome Analysis of Chemically-Induced Sensory Neuron Ablation in Zebrafish Jane A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electromagnetic Field and TGF-Β Enhance the Compensatory
www.nature.com/scientificreports OPEN Electromagnetic feld and TGF‑β enhance the compensatory plasticity after sensory nerve injury in cockroach Periplaneta americana Milena Jankowska1, Angelika Klimek1, Chiara Valsecchi2, Maria Stankiewicz1, Joanna Wyszkowska1* & Justyna Rogalska1 Recovery of function after sensory nerves injury involves compensatory plasticity, which can be observed in invertebrates. The aim of the study was the evaluation of compensatory plasticity in the cockroach (Periplaneta americana) nervous system after the sensory nerve injury and assessment of the efect of electromagnetic feld exposure (EMF, 50 Hz, 7 mT) and TGF‑β on this process. The bioelectrical activities of nerves (pre‑and post‑synaptic parts of the sensory path) were recorded under wind stimulation of the cerci before and after right cercus ablation and in insects exposed to EMF and treated with TGF‑β. Ablation of the right cercus caused an increase of activity of the left presynaptic part of the sensory path. Exposure to EMF and TGF‑β induced an increase of activity in both parts of the sensory path. This suggests strengthening efects of EMF and TGF‑β on the insect ability to recognize stimuli after one cercus ablation. Data from locomotor tests proved electrophysiological results. The takeover of the function of one cercus by the second one proves the existence of compensatory plasticity in the cockroach escape system, which makes it a good model for studying compensatory plasticity. We recommend further research on EMF as a useful factor in neurorehabilitation. Injuries in the nervous system caused by acute trauma, neurodegenerative diseases or even old age are hard to reverse and represent an enormous challenge for modern medicine. -

Peripheral Nerve Regeneration and Muscle Reinnervation
International Journal of Molecular Sciences Review Peripheral Nerve Regeneration and Muscle Reinnervation Tessa Gordon Department of Surgery, University of Toronto, Division of Plastic Reconstructive Surgery, 06.9706 Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected]; Tel.: +1-(416)-813-7654 (ext. 328443) or +1-647-678-1314; Fax: +1-(416)-813-6637 Received: 19 October 2020; Accepted: 10 November 2020; Published: 17 November 2020 Abstract: Injured peripheral nerves but not central nerves have the capacity to regenerate and reinnervate their target organs. After the two most severe peripheral nerve injuries of six types, crush and transection injuries, nerve fibers distal to the injury site undergo Wallerian degeneration. The denervated Schwann cells (SCs) proliferate, elongate and line the endoneurial tubes to guide and support regenerating axons. The axons emerge from the stump of the viable nerve attached to the neuronal soma. The SCs downregulate myelin-associated genes and concurrently, upregulate growth-associated genes that include neurotrophic factors as do the injured neurons. However, the gene expression is transient and progressively fails to support axon regeneration within the SC-containing endoneurial tubes. Moreover, despite some preference of regenerating motor and sensory axons to “find” their appropriate pathways, the axons fail to enter their original endoneurial tubes and to reinnervate original target organs, obstacles to functional recovery that confront nerve surgeons. Several surgical manipulations in clinical use, including nerve and tendon transfers, the potential for brief low-frequency electrical stimulation proximal to nerve repair, and local FK506 application to accelerate axon outgrowth, are encouraging as is the continuing research to elucidate the molecular basis of nerve regeneration. -

How Is the Brain Organized?
p CHAPTER 2 How Is the Brain Organized? An Overview of Brain Structure The Functional Organization Brain Terminology of the Brain The Brain’s Surface Features Principle 1: The Sequence of Brain Processing The Brain’s Internal Features Is “In Integrate Out” Microscopic Inspection: Cells and Fibers Principle 2: Sensory and Motor Divisions Exist Focus on Disorders: Meningitis and Throughout the Nervous System Encephalitis Principle 3: The Brain’s Circuits Are Crossed Focus on Disorders: Stroke Principle 4: The Brain Is Both Symmetrical and Asymmetrical Principle 5: The Nervous System Works A Closer Look at Neuroanatomy Through Excitation and Inhibition The Cranial Nervous System Principle 6: The Central Nervous System Has The Spinal Nervous System Multiple Levels of Function The Internal Nervous System Principle 7: Brain Systems Are Organized Both Focus on Disorders: Magendie, Bell, and Bell’s Hierarchically and in Parallel Palsy Principle 8: Functions in the Brain Are Both Localized and Distributed A. Klehr / Stone Images Micrograph: Carolina Biological Supply Co. / Phototake 36 I p hen buying a new car, people first inspect the In many ways, examining a brain for the first time is outside carefully, admiring the flawless finish similar to looking under the hood of a car. We have a vague W and perhaps even kicking the tires. Then they sense of what the brain does but no sense of how the parts open the hood and examine the engine, the part of the car that we see accomplish these tasks. We may not even be responsible for most of its behavior—and misbehavior. able to identify many of the parts. -
Nervous System Central Nervous System Peripheral Nervous System
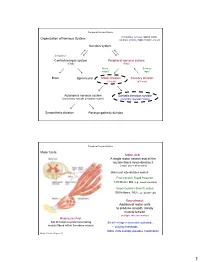
Peripheral Nervous System Involuntary reflexes (spinal cord); Organization of Nervous System: voluntary actions (higher brain centers) Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (efferent) (afferent) Autonomic nervous system Somatic nervous system (involuntary; smooth & cardiac muscle) (voluntary; skeletal muscle) Sympathetic division Parasympathetic division Peripheral Nervous System Motor Units: Motor Unit: A single motor neuron and all the muscle fibers innervated by it (motor unit = all-or-none) Motor unit size dictates control: Fine Control / Rapid Reaction: 1-10 fibers / MU (e.g., ocular muscles) Gross Control / Slow Reaction: 1000’s fibers / MU (e.g., quadriceps) Recruitment: Addition of motor units to produce smooth, steady muscle tension (multiple fiber summation) Motoneuron Pool: Set of motor neurons innervating Small large motor units activated… muscle fibers within the same muscle • Varying thresholds Motor units overlap; provides coordination Marieb & Hoehn – Figure 9.13 1 Peripheral Nervous System Types of Motor Neurons: 1) Alpha () motor neurons: • Give rise to large Type A alpha (A) motor nerve fibers (~ 14 µm diameter) • Innervate extrafusal skeletal muscle fibers (generate force) 2) Gamma () motor neurons: • Give rise to small Type A gamma (Aγ) motor nerve fibers (~ 5 µm diameter) • Innervate intrafusal muscle fibers (small, specialized fibers – muscle spindle) What is the length of the muscle? Proper -

The Nervous System Unit One: Source
UNIT ONE THE NERVOUS SYSTEM Unit One: The Nervous System UNC-CH Brain Explorers May be reproduced for non-profit educational use only. Please credit source. 3 UNIT ONE THE NERVOUS SYSTEM Unit One: The Nervous System UNC-CH Brain Explorers May be reproduced for non-profit educational use only. Please credit source. 4 THE NERVOUS SYSTEM UNIFYING SUMMARY KEY POINTS CONCEPTS FULL BODY TRACING • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • Models help us understand and In the Full Body Tracing students • All our thooughts, movements, explain the world.* work together as a class to create sensations and emotions are • Systems are made of parts which a model of the nervous system. controlled by the nervous system. connect to create the whole.* The outline of a student’s body is • We have a Central Nervous • The brain receives informational traced on a large piece of paper. System (brain and spinal cord) signals from all parts of the body. A cardboard cut out of the brain and Peripheral Nervous System The brain sends signals to all parts and spinal cord are placed in the (nerves extending from the spinal of the body to influence what they outline to represent the central cord to limbs, trunk, face, organs do.* nervous system. Two colors of and throughout.) • Humans have systems for yarn, representing the motor and • Sensory nerves communicate digestion, circulation, movement sensory nerves, are used to create information from the body to the and coordination. These systems motor and sensory pathways of brain and motor nerves, from the interact with one another.* the peripheral nervous system. brain to the body. -

Traumatic Injury to Peripheral Nerves
AAEM MINIMONOGRAPH 28 ABSTRACT: This article reviews the epidemiology and classification of traumatic peripheral nerve injuries, the effects of these injuries on nerve and muscle, and how electrodiagnosis is used to help classify the injury. Mecha- nisms of recovery are also reviewed. Motor and sensory nerve conduction studies, needle electromyography, and other electrophysiological methods are particularly useful for localizing peripheral nerve injuries, detecting and quantifying the degree of axon loss, and contributing toward treatment de- cisions as well as prognostication. © 2000 American Association of Electrodiagnostic Medicine. Published by John Wiley & Sons, Inc. Muscle Nerve 23: 863–873, 2000 TRAUMATIC INJURY TO PERIPHERAL NERVES LAWRENCE R. ROBINSON, MD Department of Rehabilitation Medicine, University of Washington, Seattle, Washington 98195 USA EPIDEMIOLOGY OF PERIPHERAL NERVE TRAUMA company central nervous system trauma, not only Traumatic injury to peripheral nerves results in con- compounding the disability, but making recognition siderable disability across the world. In peacetime, of the peripheral nerve lesion problematic. Of pa- peripheral nerve injuries commonly result from tients with peripheral nerve injuries, about 60% have 30 trauma due to motor vehicle accidents and less com- a traumatic brain injury. Conversely, of those with monly from penetrating trauma, falls, and industrial traumatic brain injury admitted to rehabilitation accidents. Of all patients admitted to Level I trauma units, 10 to 34% have associated peripheral nerve 7,14,39 centers, it is estimated that roughly 2 to 3% have injuries. It is often easy to miss peripheral nerve peripheral nerve injuries.30,36 If plexus and root in- injuries in the setting of central nervous system juries are also included, the incidence is about 5%.30 trauma. -

Peripheral Nerve Injury: Principles for Repair and Regeneration M.F
Send Orders for Reprints to [email protected] The Open Orthopaedics Journal, 2014, 8, (Suppl 1: M10) 199-203 199 Open Access Peripheral Nerve Injury: Principles for Repair and Regeneration M.F. Griffin1, M. Malahias1, S. Hindocha*,2 and Wasim S. Khan3 1Plastic Surgery Department, Good Hope Hospital, West Midlands, B75 7RR, UK 2Plastic Surgery Department, Whiston Hospital, Liverpool, L35 5DR, UK 3University College London Institute of Orthopaedics & Musculoskeletal Sciences, Royal National Orthopaedic Hospital, Stanmore, London, HA7 4LP, UK Abstract: Peripheral Nerve Injuries are one of the most common causes of hand dysfunction caused by upper limb trauma but still current management has remained suboptimal. This review aims to explain the traditional view of pathophysiology of nerve repair and also describe why surgical management is still inadequate in using the new biological research that has documented the changes that occur after the nerve injury, which, could cause suboptimal clinical outcomes. Subsequently presentation and diagnosis will be described for peripheral nerve injuries. When traditional surgical repair using end-to-end anastomosis is not adequate nerve conduits are required with the gold standard being the autologous nerve. Due to associated donor site morbidity and poor functional outcome documented with autologous nerve repair several new advancements for alternatives to bridge the gap are being investigated. We will summarise the new and future advancements of non-biological and biological replacements as well as gene therapy, which are being considered as the alternatives for peripheral nerve repair. Keywords: Anastamosis, biological replacements, clinical outcome, nerve repair, peripheral nerves. INTRODUCTION perineurium; which contributes to nerve strength and maintains the intra-fascicular pressure. -

How Does the Brain Produce Movement?
p CHAPTER 10 How Does the Brain Produce Movement? The Hierarchical Control The Basal Ganglia and of Movement the Cerebellum The Forebrain and Movement Initiation The Basal Ganglia and Movement Force The Brainstem and Species-Typical Movement Focus on Disorders: Tourette’s Syndrome Focus on Disorders: Autism The Cerebellum and Movement Skill The Spinal Cord and Movement Execution Focus on Disorders: Paraplegia The Organization of the Somatosensory System The Organization of the Somatosensory Receptors and Sensory Perception Motor System Dorsal-Root Ganglion Neurons The Motor Cortex The Somatosensory Pathways to the Brain The Corticospinal Tracts Spinal-Cord Responses to Somatosensory Input The Motor Neurons The Vestibular System and Balance The Control of Muscles Exploring the Somatosensory The Motor Cortex and Skilled System Movements The Somatosensory Homunculus Investigating Neural Control of Skilled Movements The Effects of Damage to the Somatosensory Cortex The Control of Skilled Movements in Other Species The Somatosensory Cortex and Complex How Motor Cortex Damage Affects Skilled Movement Movements Kevork Djansezian/AP Photo Micrograph: Dr. David Scott/Phototake 354 I p amala is a female Indian elephant that lives at the In one way, however, Kamala uses this versatile trunk zoo in Calgary, Canada. Her trunk, which is really very unusually for an elephant (Onodera & Hicks, 1999). K just a greatly extended upper lip and nose, con- She is one of only a few elephants in the world that paints sists of about 2000 fused muscles. A pair of nostrils runs its with its trunk (Figure 10-1). Like many artists, she paints length and fingerlike projections are located at its tip. -

Basic Nerve Conduction Studies Holli A
Basic Nerve Conduction Studies Holli A. Horak, MD University of Arizona August 2015 Introduction Review nerve physiology/ anatomy Purpose of testing Study design Motor NCS Sensory NCS Mixed NCS Interpretation Technical considerations Summary Anatomy Motor Neuron Axon Myelin Neuromuscular Junction Muscle fibers Anatomy Dorsal Root ganglion: Bipolar Nerve cell One projection central Dorsal column Other axon distal Sensory end organ Myelinated: different degrees Anatomy Neurons AHC DRG Roots Rami Ventral Rami: Plexus Dorsal Rami: Paraspinals Anatomy Certain nerves are routinely studied Location Size Important pathology Ease of evaluation Some are less often studied Some are rarely studied Study Design Answer the clinical question Not just routine Specifically choose nerve evaluation needed Motor NCS Sensory NCS Repetitive stimulation Other (mixed study) Least number of NCS needed to answer the clinical question I.e.. CTS Purpose of testing Neuropathy Other conditions Focal Radiculopathy Carpal Tunnel Syndrome (CTS) Peroneal neuropathy Ulnar neuropathy Neuromuscular junction defects Generalized Diabetic Neuropathy Myasthenia Gravis Guillain Barre syndrome (GBS) LEMS Axonal Motor Neuron Disease Diabetic Neuropathy ALS Nerve transection Demyelinating Sensory Neuronopathy GBS Sjogren’s disease CTS Motor nerve conduction studies Larger More reproducible Troubleshooting is easier Why? Compound Muscle Action Potential Muscle amplifies the response Stimulate nerve axons Causes -

PERIPHERAL NEUROPATHY Ralph F
PERIPHERAL NEUROPATHY Ralph F. Józefowicz, MD Peripheral neuropathy is a general term for any disorder affecting the peripheral nerves. Since peripheral neuropathy can be caused by numerous factors, an investigation into the cause of the neuropathy should be undertaken as soon as the diagnosis of neuropathy is made. CLINICAL FEATURES Symptoms Since the peripheral nervous system consists of motor, sensory and autonomic nerves, symptoms can fall into all three of these categories. • Sensory symptoms include distal dysesthesias, pain and numbness. A characteristic pattern of numbness is one in which the distal portions of the nerves are first affected, the so-called "stocking-glove" pattern. This pattern occurs because nerve fibers are affected according to length of axon, without regard to root or nerve trunk distribution. • Motor symptoms include weakness, which once again is distal, and typically involves extensor groups rather than flexor groups of muscles. • Autonomic dysfunction is common and includes orthostasis, impotence in males and gastroparesis. Signs Signs of peripheral neuropathy also include sensory, motor and autonomic components. • Sensory disturbance is manifest as distal loss of pin, temperature and vibratory perception as well as proprioception. Initial signs are frequently confined to the toes and feet. A positive Romberg sign is frequently present due to proprioceptive loss in the lower extremities. • Motor signs include distal weakness, primarily in extensor groups, and most prominent in the lower extremities initially. Distal muscles are often atrophic, and one should carefully assess the bulk of the extensor digitorum brevis muscles in the feet and of the intrinsic muscles of the hands. Muscle tone is reduced and often is flaccid. -

Stretch Reflex and Golgi Tendon Reflex
Stretch reflex and Golgi Tendon Reflex Prof. Faten zakareia Physiology Department , College of Medicine , King Saud University 2016 • Objectives: Upon completion of this lecture, students should be able to know and explain : - The definition and components of stretch reflex - The structure , innervations and function of the muscle spindle -Sensory primary and secondary (flower-spray) sensory afferent fibres of muscle spindle, Intrafusal muscle fibers(nuclear bag &nuclear chain fibers) - The Dynamic gamma efferent and Trail endings discharge and their functional role - What is meant by static and dynamic stretch reflex& damping mechanism - Muscle tone and its abnormalities - The spinal and supraspinal regulation of the stretch reflex - the inverse stretch reflex (golgi tendon reflex)and its function Textbook/Guyton & Hall Reference book/Ganong review of medical physiology THE STRETCH REFLEX REFLEX STRETCH (MYOTACTIC) REFLEX https://musom.marshall.edu/anatomy/grosshom/allppt/pdf/Spinalreflexes.pdf CLINICAL TEST | RAPID STRETCH OF MUSCLE (TAP ON MUSCLE TENDON) STIMULUS RESPONSE STRETCHED MUSCLE CONTRACT RAPIDLY (I.E. KNEE JERK) SENSORY MUSCLE SPINDLE PRIMARY RECEPTOR SYNAPSES MONOSYNAPTIC INVOLVED EFFECTS ON CONTRACTS (+) SAME MUSCLE AND SYNERGISTIC MUSCLES MUSCLE OTHER EFFECTS RELAXES (-) ANTAGONISTIC MUSCLE FUNCTION AIDS IN MAINTAINING POSTURE, avoid muscle rupture,counters sudden loads 3 What is the Stretch Reflex ? • It is reflex contraction of muscle resulting from stimulation of the muscle spindle by stretch Muscle spindle is the receptor -

The Nervous System School House RockNervous System Brainpop Nervous System
The Nervous System School House RockNervous System Brainpop Nervous System Central Nervous System: Made up of the brain and the spinal cord Peripheral Nervous System : Made up of the nerves not found in the central nervous system a.) somatic: control voluntary actions. b.) autonomic: control involuntary actions. Phineas Gage Frontal Lobe and Personality 1 Senses receive a stimuli from the environment through a sensory nerve, the nerves carry the message to the spinal cord and up to the brain. The brain interprets the message and sends a message back to the body through motor nerves. The body then reacts to the stimuli. Stimuli Sensory Nerves Spinal Cord (Interneurons) Brain Spinal Cord Motor Nerves Stimuli: A message from the environment. Senses: sight, smell, taste, touch, and hearing Optical illusions 2 Central Nervous System: Made up of the brain and the spinal cord Peripheral Nervous System : Made up of the nerves not found in the central nervous system a.) somatic: control voluntary actions. b.) autonomic: control involuntary actions. Senses receive a stimuli from the environment through a sensory nerve, the nerves carry the message to the spinal cord and up to the brain. The brain interprets the message and sends a message back to the body through motor nerves. The body then reacts to the stimuli. Stimuli Sensory Nerves Spinal Cord (Interneurons) Brain Spinal Cord Motor Nerves Stimuli: A message from the environment. Senses: sight, smell, taste, touch, and hearing 3 Bill Nye On The Brain Part 1 4 BrainpopBrain Cerebrum: Made up of four different lobes (frontal, parietal, occipital, and temporal).