Stretch Reflex and Golgi Tendon Reflex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -
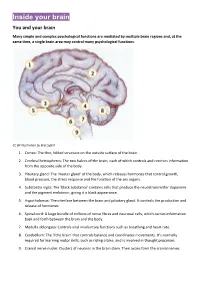
Inside Your Brain You and Your Brain
Inside your brain You and your brain Many simple and complex psychological functions are mediated by multiple brain regions and, at the same time, a single brain area may control many psychological functions. CC BY Illustration by Bret Syfert 1. Cortex: The thin, folded structure on the outside surface of the brain. 2. Cerebral hemispheres: The two halves of the brain, each of which controls and receives information from the opposite side of the body. 3. Pituitary gland: The ‘master gland’ of the body, which releases hormones that control growth, blood pressure, the stress response and the function of the sex organs. 4. Substantia nigra: The ‘black substance’ contains cells that produce the neurotransmitter dopamine and the pigment melatonin, giving it a black appearance. 5. Hypothalamus: The interface between the brain and pituitary gland. It controls the production and release of hormones. 6. Spinal cord: A large bundle of millions of nerve fibres and neuronal cells, which carries information back and forth between the brain and the body. 7. Medulla oblongata: Controls vital involuntary functions such as breathing and heart rate. 8. Cerebellum: The ‘little brain’ that controls balance and coordinates movements. It’s normally required for learning motor skills, such as riding a bike, and is involved in thought processes. 9. Cranial nerve nuclei: Clusters of neurons in the brain stem. Their axons form the cranial nerves. Your brain underpins who you are. It stores your knowledge and memories, gives you the capacity for thought and emotion, and enables you to control your body. The brain is just one part of the nervous system. -

Electromagnetic Field and TGF-Β Enhance the Compensatory
www.nature.com/scientificreports OPEN Electromagnetic feld and TGF‑β enhance the compensatory plasticity after sensory nerve injury in cockroach Periplaneta americana Milena Jankowska1, Angelika Klimek1, Chiara Valsecchi2, Maria Stankiewicz1, Joanna Wyszkowska1* & Justyna Rogalska1 Recovery of function after sensory nerves injury involves compensatory plasticity, which can be observed in invertebrates. The aim of the study was the evaluation of compensatory plasticity in the cockroach (Periplaneta americana) nervous system after the sensory nerve injury and assessment of the efect of electromagnetic feld exposure (EMF, 50 Hz, 7 mT) and TGF‑β on this process. The bioelectrical activities of nerves (pre‑and post‑synaptic parts of the sensory path) were recorded under wind stimulation of the cerci before and after right cercus ablation and in insects exposed to EMF and treated with TGF‑β. Ablation of the right cercus caused an increase of activity of the left presynaptic part of the sensory path. Exposure to EMF and TGF‑β induced an increase of activity in both parts of the sensory path. This suggests strengthening efects of EMF and TGF‑β on the insect ability to recognize stimuli after one cercus ablation. Data from locomotor tests proved electrophysiological results. The takeover of the function of one cercus by the second one proves the existence of compensatory plasticity in the cockroach escape system, which makes it a good model for studying compensatory plasticity. We recommend further research on EMF as a useful factor in neurorehabilitation. Injuries in the nervous system caused by acute trauma, neurodegenerative diseases or even old age are hard to reverse and represent an enormous challenge for modern medicine. -

GDE2 Regulates Subtype-Specific Motor Neuron Generation Through
Neuron Article GDE2 Regulates Subtype-Specific Motor Neuron Generation through Inhibition of Notch Signaling Priyanka Sabharwal,1 Changhee Lee,1 Sungjin Park,1 Meenakshi Rao,1,2 and Shanthini Sockanathan1,* 1The Solomon Snyder Department of Neuroscience, The Johns Hopkins University School of Medicine, PCTB1004, 725 N. Wolfe Street, Baltimore, MD 21205, USA 2Present address: Department of Pediatrics, Children’s Hospital Boston, 300 Longwood Avenue, Boston, MA 02115, USA *Correspondence: [email protected] DOI 10.1016/j.neuron.2011.07.028 SUMMARY which is innervated by specific groups of motor neurons. Indi- vidual motor neuron groups are highly organized in terms of their The specification of spinal interneuron and motor cell body distribution, projection patterns, and function and neuron identities initiates within progenitor cells, consist of force-generating alpha motor neurons that innervate while motor neuron subtype diversification is regu- extrafusal muscle fibers and stretch-sensitive gamma motor lated by hierarchical transcriptional programs imple- neurons that innervate intrafusal muscle fibers of the muscle mented postmitotically. Here we find that mice lack- spindles (Dasen and Jessell, 2009; reviewed in Kanning et al., ing GDE2, a six-transmembrane protein that triggers 2010). The integration of input from both alpha and gamma motor neurons is essential for coordinated motor movement to motor neuron generation, exhibit selective losses occur (Kanning et al., 2010). of distinct motor neuron subtypes, specifically in How is diversity engendered in developing motor neurons? defined subsets of limb-innervating motor pools All motor neurons initially derive from ventral progenitor cells that correlate with the loss of force-generating alpha that are specified to become Olig2+ motor neuron progenitors motor neurons. -

Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems
cells Review Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems Naoki Abe 1, Tasuku Nishihara 1,*, Toshihiro Yorozuya 1 and Junya Tanaka 2 1 Department of Anesthesia and Perioperative Medicine, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] (N.A.); [email protected] (T.Y.) 2 Department of Molecular and cellular Physiology, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-89-960-5383; Fax: +81-89-960-5386 Received: 4 August 2020; Accepted: 17 September 2020; Published: 21 September 2020 Abstract: Microglia, the immunocompetent cells in the central nervous system (CNS), have long been studied as pathologically deteriorating players in various CNS diseases. However, microglia exert ameliorating neuroprotective effects, which prompted us to reconsider their roles in CNS and peripheral nervous system (PNS) pathophysiology. Moreover, recent findings showed that microglia play critical roles even in the healthy CNS. The microglial functions that normally contribute to the maintenance of homeostasis in the CNS are modified by other cells, such as astrocytes and infiltrated myeloid cells; thus, the microglial actions on neurons are extremely complex. For a deeper understanding of the pathophysiology of various diseases, including those of the PNS, it is important to understand microglial functioning. In this review, we discuss both the favorable and unfavorable roles of microglia in neuronal survival in various CNS and PNS disorders. We also discuss the roles of blood-borne macrophages in the pathogenesis of CNS and PNS injuries because they cooperatively modify the pathological processes of resident microglia. -

Reduction of Ephrin-A5 Aggravates Disease Progression in Amyotrophic
Rué et al. Acta Neuropathologica Communications (2019) 7:114 https://doi.org/10.1186/s40478-019-0759-6 RESEARCH Open Access Reduction of ephrin-A5 aggravates disease progression in amyotrophic lateral sclerosis Laura Rué1,2 , Patrick Oeckl3, Mieke Timmers1,2, Annette Lenaerts1,2, Jasmijn van der Vos1,2, Silke Smolders1,2, Lindsay Poppe1,2, Antina de Boer1,2, Ludo Van Den Bosch1,2, Philip Van Damme1,2,4, Jochen H. Weishaupt3, Albert C. Ludolph3, Markus Otto3, Wim Robberecht1,4 and Robin Lemmens1,2,4* Abstract Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that affects motor neurons in the brainstem, spinal cord and motor cortex. ALS is characterized by genetic and clinical heterogeneity, suggesting the existence of genetic factors that modify the phenotypic expression of the disease. We previously identified the axonal guidance EphA4 receptor, member of the Eph-ephrin system, as an ALS disease-modifying factor. EphA4 genetic inhibition rescued the motor neuron phenotype in zebrafish and a rodent model of ALS. Preventing ligands from binding to the EphA4 receptor also successfully improved disease, suggesting a role for EphA4 ligands in ALS. One particular ligand, ephrin-A5, is upregulated in reactive astrocytes after acute neuronal injury and inhibits axonal regeneration. Moreover, it plays a role during development in the correct pathfinding of motor axons towards their target limb muscles. We hypothesized that a constitutive reduction of ephrin-A5 signalling would benefit disease progression in a rodent model for ALS. We discovered that in the spinal cord of control and symptomatic ALS mice ephrin-A5 was predominantly expressed in neurons. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Gamma Motor Neurons Survive and Exacerbate Alpha Motor Neuron Degeneration In
Gamma motor neurons survive and exacerbate alpha PNAS PLUS motor neuron degeneration in ALS Melanie Lalancette-Heberta,b, Aarti Sharmaa,b, Alexander K. Lyashchenkoa,b, and Neil A. Shneidera,b,1 aCenter for Motor Neuron Biology and Disease, Columbia University, New York, NY 10032; and bDepartment of Neurology, Columbia University, New York, NY 10032 Edited by Rob Brownstone, University College London, London, United Kingdom, and accepted by Editorial Board Member Fred H. Gage October 27, 2016 (received for review April 4, 2016) The molecular and cellular basis of selective motor neuron (MN) the muscle spindle and control the sensitivity of spindle afferent vulnerability in amyotrophic lateral sclerosis (ALS) is not known. In discharge (15); beta (β) skeletofusimotor neurons innervate both genetically distinct mouse models of familial ALS expressing intra- and extrafusal muscle (16). In addition to morphological mutant superoxide dismutase-1 (SOD1), TAR DNA-binding protein differences, distinct muscle targets, and the absence of primary 43 (TDP-43), and fused in sarcoma (FUS), we demonstrate selective afferent (IA)inputsonγ-MNs, these functional MN subtypes also degeneration of alpha MNs (α-MNs) and complete sparing of differ in their trophic requirements, and γ-MNs express high levels gamma MNs (γ-MNs), which selectively innervate muscle spindles. of the glial cell line-derived neurotropic factor (GDNF) receptor Resistant γ-MNs are distinct from vulnerable α-MNs in that they Gfrα1 (17). γ-MNs are also molecularly distinguished by the ex- lack synaptic contacts from primary afferent (IA) fibers. Elimination pression of other selective markers including the transcription α of these synapses protects -MNs in the SOD1 mutant, implicating factor Err3 (18), Wnt7A (19), the serotonin receptor 1d (5-ht1d) this excitatory input in MN degeneration. -

Microglia and C9orf72 in Neuroinflammation and ALS and Frontotemporal Dementia
Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia Deepti Lall, Robert H. Baloh J Clin Invest. 2017;127(9):3250-3258. https://doi.org/10.1172/JCI90607. Review Series Amyotrophic lateral sclerosis (ALS) is a degenerative disorder that is characterized by loss of motor neurons and shows clinical, pathological, and genetic overlap with frontotemporal dementia (FTD). Activated microglia are a universal feature of ALS/FTD pathology; however, their role in disease pathogenesis remains incompletely understood. The recent discovery that ORF 72 on chromosome 9 (C9orf72), the gene most commonly mutated in ALS/FTD, has an important role in myeloid cells opened the possibility that altered microglial function plays an active role in disease. This Review highlights the contribution of microglia to ALS/FTD pathogenesis, discusses the connection between autoimmunity and ALS/FTD, and explores the possibility that C9orf72 and other ALS/FTD genes may have a “dual effect” on both neuronal and myeloid cell function that could explain a shared propensity for altered systemic immunity and neurodegeneration. Find the latest version: https://jci.me/90607/pdf REVIEW SERIES: GLIA AND NEURODEGENERATION The Journal of Clinical Investigation Series Editors: Marco Colonna and David Holtzmann Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia Deepti Lall1 and Robert H. Baloh1,2 1Board of Governors Regenerative Medicine Institute and 2Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, California, USA. Amyotrophic lateral sclerosis (ALS) is a degenerative disorder that is characterized by loss of motor neurons and shows clinical, pathological, and genetic overlap with frontotemporal dementia (FTD). Activated microglia are a universal feature of ALS/FTD pathology; however, their role in disease pathogenesis remains incompletely understood. -

ARIA Is Concentrated in Nerve Terminals at Neuromuscular Junctions and at Other Synapses
The Journal of Neuroscience, September 1995, 15(g): 6124-6136 ARIA Is Concentrated in Nerve Terminals at Neuromuscular Junctions and at Other Synapses Alfred W. Sandrock, Jr.,i,2 Andrew D. J. Goodearl,’ Qin-Wei Yin,’ David Chang,3 and Gerald D. Fischbachl ‘Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115, “Department of Neurology, Massachusetts General Hosoital, Boston, Massachusetts 02114, and 3Amgen, Inc., Thousand Oaks, California 91320 Skeletal muscle ACh receptors (AChRs) accumulate at neu- natal maturation of AChRs at the neuromuscular junction, which romuscular junctions (nmjs) at least partly because of the results in the replacement of y-subunit-containing receptors with selective induction of AChR subunit genes in subsynaptic those containing E- subunits (Brenner and Sakmann, 1978; Bren- myotube nuclei by the motor nerve terminal. Additionally, ner et al., 1990; Martinou and Merlie, 1991). ARIA (for ACh mammalian AChRs undergo a postnatal change in subunit receptor inducing activity), which was purified from chicken composition from embryonic (cw.&S) to adult (@eS) brains based on its ability to stimulate the synthesis of AChRs forms, a switch that also depends on innervation. ARIA, a in skeletal muscle (Falls et al., 1993), may mediate the ability protein purified from chicken brains based on its ability to of the motor neuron to orchestrate these developmental events. induce AChR synthesis in primary chick muscle cells, is a ARIA mRNA is concentrated in chick and rat motor neurons strong candidate for being the molecule responsible for (Falls et al., 1993; Corfas et al., 1995), and, in both species, is these early developmental events. -

Mitigation of ALS Pathology by Neuron-Specific Inhibition of Nuclear Factor Kappa B Signaling
The Journal of Neuroscience, June 24, 2020 • 40(26):5137–5154 • 5137 Neurobiology of Disease Mitigation of ALS Pathology by Neuron-Specific Inhibition of Nuclear Factor Kappa B Signaling Kallol Dutta,1 Sai Sampath Thammisetty,1 Hejer Boutej,1 Christine Bareil,1 and Jean-Pierre Julien1,2 1CERVO Brain Research Centre, Québec City, Québec G1J 2G3, Canada, and 2Department of Psychiatry and Neuroscience, Université Laval, Québec City, Québec G1V 0A6, Canada To investigate the role of neuronal NF-jB activity in pathogenesis of amyotrophic lateral sclerosis (ALS), we generated trans- genic mice with neuron-specific expression of a super-repressor form of the NF-jB inhibitor (IjBa-SR), which were then crossed with mice of both sexes, expressing ALS-linked gene mutants for TAR DNA-binding protein (TDP-43) and superoxide dismutase 1 (SOD1). Remarkably, neuronal expression of IjBa-SR transgene in mice expressing TDP-43A315T or TDP-43G348C mice led to a decrease in cytoplasmic to nuclear ratio of human TDP-43. The mitigation of TDP-43 neuropathology by IjBa-SR, which is likely due to an induction of autophagy, was associated with amelioration of cognitive and motor deficits as well as reduc- tion of motor neuron loss and gliosis. Neuronal suppression of NF-jB activity in SOD1G93A mice also resulted in neuroprotection with reduction of misfolded SOD1 levels and significant extension of life span. The results suggest that neuronal NF-jB signaling constitutes a novel therapeutic target for ALS disease and related disorders with TDP-43 proteinopathy. Key words: amyotrophic lateral sclerosis; frontotemporal dementia; IjB suppressor; NF-jB; superoxide dismutase; TDP-43 Significance Statement This study reports that neuron-specific expression of IkB super-repressor mitigated behavioral and pathologic changes in transgenic mouse models of amyotrophic lateral sclerosis expressing mutant forms of either Tar DNA-binding protein 43 or superoxide dismutase. -

Perineurial Glia Require Notch Signaling During Motor Nerve Development but Not Regeneration
The Journal of Neuroscience, March 6, 2013 • 33(10):4241–4252 • 4241 Development/Plasticity/Repair Perineurial Glia Require Notch Signaling during Motor Nerve Development but Not Regeneration Laura A. Binari, Gwendolyn M. Lewis, and Sarah Kucenas Department of Biology, University of Virginia, Charlottesville, Virginia 22904 Motor nerves play the critical role of shunting information out of the CNS to targets in the periphery. Their formation requires the coordinated development of distinct cellular components, including motor axons and the Schwann cells and perineurial glia that en- sheath them. During nervous system assembly, these glial cells must migrate long distances and terminally differentiate, ensuring the efficient propagation of action potentials. Although we know quite a bit about the mechanisms that control Schwann cell development during this process, nothing is known about the mechanisms that mediate the migration and differentiation of perineurial glia. Using in vivo imaging in zebrafish, we demonstrate that Notch signaling is required for both perineurial migration and differentiation during nerve formation, but not regeneration. Interestingly, loss of Notch signaling in perineurial cells also causes a failure of Schwann cell differentiation, demonstrating that Schwann cells require perineurial glia for aspects of their own development. These studies describe a novel mechanism that mediates multiple aspects of perineurial development and reveal the critical importance of perineurial glia for Schwann cell maturation and nerve formation. Introduction independent. However, to date, nothing is known about these A fundamental goal in neuroscience is to understand what con- nonaxonal mechanisms. trols cell migration. Unlike other organ systems where cells are In zebrafish, perineurial glia originate from precursors in restricted to a discrete space, peripheral glia must migrate long the floor plate (p3 domain) of the spinal cord, migrate out of distances from their origin to their target.