Peripheral Nerve Injury: Principles for Repair and Regeneration M.F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electromagnetic Field and TGF-Β Enhance the Compensatory
www.nature.com/scientificreports OPEN Electromagnetic feld and TGF‑β enhance the compensatory plasticity after sensory nerve injury in cockroach Periplaneta americana Milena Jankowska1, Angelika Klimek1, Chiara Valsecchi2, Maria Stankiewicz1, Joanna Wyszkowska1* & Justyna Rogalska1 Recovery of function after sensory nerves injury involves compensatory plasticity, which can be observed in invertebrates. The aim of the study was the evaluation of compensatory plasticity in the cockroach (Periplaneta americana) nervous system after the sensory nerve injury and assessment of the efect of electromagnetic feld exposure (EMF, 50 Hz, 7 mT) and TGF‑β on this process. The bioelectrical activities of nerves (pre‑and post‑synaptic parts of the sensory path) were recorded under wind stimulation of the cerci before and after right cercus ablation and in insects exposed to EMF and treated with TGF‑β. Ablation of the right cercus caused an increase of activity of the left presynaptic part of the sensory path. Exposure to EMF and TGF‑β induced an increase of activity in both parts of the sensory path. This suggests strengthening efects of EMF and TGF‑β on the insect ability to recognize stimuli after one cercus ablation. Data from locomotor tests proved electrophysiological results. The takeover of the function of one cercus by the second one proves the existence of compensatory plasticity in the cockroach escape system, which makes it a good model for studying compensatory plasticity. We recommend further research on EMF as a useful factor in neurorehabilitation. Injuries in the nervous system caused by acute trauma, neurodegenerative diseases or even old age are hard to reverse and represent an enormous challenge for modern medicine. -

Wallerian Degeneration and Inflammation in Rat Peripheral Nerve Detected by in Vivo MR Imaging
741 Wallerian Degeneration and Inflammation in Rat Peripheral Nerve Detected by in Vivo MR Imaging DavidS. Titelbaum 1 To investigate the role of MR imaging in wallerian degeneration, a series of animal Joel L. Frazier 2 models of increasingly complex peripheral nerve injury were studied by in vivo MR. Robert I. Grossman 1 Proximal tibial nerves in brown Norway rats were either crushed, transected (neurotomy), Peter M. Joseph 1 or transected and grafted with Lewis rat (allograft) or brown Norway (isograft) donor Leonard T. Yu 2 nerves. The nerves distal to the site of injury were imaged at intervals of 0-54 days after surgery. Subsequent histologic analysis was obtained and correlated with MR Eleanor A. Kassab 1 3 findings. Crush injury, neurotomy, and nerve grafting all resulted in high signal intensity William F. Hickey along the course of the nerve observed on long TR/TE sequences, corresponding to 2 Don LaRossa edema and myelin breakdown from wallerian degeneration. The abnormal signal inten 4 Mark J. Brown sity resolved by 30 days after crush injury and by 45-54 days after neurotomy, when the active changes of wallerian degeneration had subsided. These changes were not seen in sham-operated rats. Our findings suggest that MR is capable of identifying traumatic neuropathy in a peripheral nerve undergoing active wallerian degeneration. The severity of injury may be reflected by the corresponding duration of signal abnormality. With the present methods, MR did not distinguish inflammatory from simple posttraumatic neuropathy. Wallerian degeneration is the axonal degeneration and loss of myelin that occurs when an axon is separated from its cell body. -
Nerve Injury After Peripheral Nerve Block: Allbest Rights Practices Reserved
PRINTER-FRIENDLY VERSION AVAILABLE AT ANESTHESIOLOGYNEWS.COM Nerve Injury After Peripheral Nerve Block: AllBest rights Practices reserved. Reproduction and Medical-Legal in whole or in part without Protection permission isStrategies prohibited. Copyright © 2015 McMahon Publishing Group unless otherwise noted. DAVID HARDMAN, MD, MBA Professor of Anesthesiology Vice Chair for Professional Affairs Department of Anesthesiology University of North Carolina at Chapel Hill Chapel Hill, North Carolina Dr. Hardman reports no relevant financial conflicts of interest. he risk for permanent or severe nerve injury after peripheral nerve blocks (PNBs) is Textremely low, irrespective of its etiology (ie, related to anesthesia, surgery or the patient). The risk inherent in a procedure should always be explicitly discussed with the patient (sidebar, page 4). In fact, it may be better to define this phenomenon ultrasound-guided axillary blocks were used, demon- as postoperative neurologic symptoms (PONS) or peri- strated a very low nerve injury rate of 0.0037% at hos- operative nerve injuries (PNI) in order to help stan- pital discharge.1-7 dardize terminology. Permanent injury rates, as defined A 2009 prospective case series involving more than by a neurologic abnormality present at or beyond 12 7,000 PNBs, conducted in Australia and New Zealand, months after the procedure, have consistently ranged demonstrated that when a postoperative neurologic from 0.029% to 0.2%, although the results of a recent symptom was diagnosed, it was 9 times more likely to multicenter Web-based survey in France, in which be due to a non–anesthesia-related cause than a nerve ANESTHESIOLOGY NEWS • JULY 2015 1 block–related cause.6 On the other hand, it is well doc- PNI rate of 1.7% in patients who received a single-injec- umented in the orthopedic and anesthesia literature tion interscalene block (ISB). -

Delayed Facial Palsy After Head Injury
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.40.4.342 on 1 April 1977. Downloaded from Journal ofNeurology, Neurosurgery, andPsychiatry, 1977, 40, 342-350 Delayed facial palsy after head injury K. PUVANENDRAN, M. VITHARANA, AND P. K. WONG From the University Department ofMedicine, and the Department ofOtorhinolaryngology, Singapore General Hospital, Singapore SUMMARY Where facial palsy follows head injury after many days, the mechanism is not clear, and there has been no detailed study on this condition. In this prospective study, an attempt is made to estimate this complication of head injury, and to study its pathogenesis, natural history, prognosis, and sequelae which differ markedly from Bell's palsy. It has a much worse prognosis and so surgical decompression should be considered early in this condition. The facial nerve is the motor cranial nerve which is studies, for prediction of prognosis at a time when most commonly affected in closed head injuries surgical intervention seems most advantageous. (Turner, 1943). In facial palsy which immediately follows a head injury, the mechanism is obvious, but Patients and methods Protected by copyright. it is not clear when the facial palsy follows the head injury after many days (Potter and Braakman, 1976). During the period May 1974-April 1975, there were Traumatic facial palsy has received much attention 6304 cases of head injury admitted to government but few authors distinguish between immediate and hospitals in Singapore. The chief criterion for delayed palsy. admission to hospital was the occurrence of traumatic Turner (1944) studied a selected group of war-time amnesia or unconsciousness, indicating concussion head injuries from a military hospital for head of the brain. -

The Role of Vagal Nerve Root Injury on Respiration Disturbances In
Original Investigation Original Received: 03.12.2013 / Accepted: 11.02.2014 DOI: 10.5137/1019-5149.JTN.9964-13.1 The Role of Vagal Nerve Root Injury on Respiration Disturbances in Subarachnoid Hemorrhage Subaraknoid Kanamada Solunum Bozuklukları Oluşmasında Vagal Sinir Kökü Hasarının Rolü Murteza CAKıR1, Canan AtALAY 2, Zeynep CAKıR3, Mucahit Emet3, Mehmet Dumlu AYDıN1, Nazan AYDıN4, Arif ONDER5, Muhammed CAlıK6 1Ataturk University, School of Medicine, Department of Neurosurgery, Erzurum, Turkey 2Ataturk University, School of Medicine, Department of Anesthesiology and Reanimation, Erzurum, Turkey 3Ataturk University, School of Medicine, Department of Emergency Medicine, Erzurum, Turkey 4Ataturk University, School of Medicine, Department of Psychiatry, Erzurum, Turkey 5Avrasya Hospital, Department of Neurosurgery, Istanbul, Turkey 6Ataturk University, School of Medicine, Department of Pathology, Erzurum, Turkey Corresponding Author: Murteza CAKır / E-mail: [email protected] ABSTRACT AIM: We examined whether there is a relationship between vagal nerve root injury and the severity of respiration disorders associated with subarachnoid hemorrhage (SAH). MaTERIAL and METHODS: This study was conducted on 20 rabbits. Experimental SAH was induced by injecting homologous blood into the cisterna magna. During the experiment, electrocardiography and respiratory rhythms were measured daily. After the experiment, any axonal injury or changes to the arterial nervorums of the vagal nerves were examined. All respiratory irregularities and vagal nerve degenerations were statistically analyzed. RESULTS: Normal respiration rate, as measured in the control group, was 30±6 bpm. In the SAH-induced group, respiration rates were initially 20±4 bpm, increasing to 40±9/min approximately ten hours later, with severe tachypneic and apneic variation. In histopathological examinations, axon density of vagal nerves was 28500±5500 in both control and sham animals, whereas axon density was 22250±3500 in survivors and 16450±2750 in dead SAH animals. -

Repairing Spinal Cord Nerves
Repairing spinal cord nerves Ronald Schnaar The Johns Hopkins School of Medicine Traumatic spinal cord injury Edwin Smith Papyrus, Egypt, circa 1500 BC Earliest medical text on battlefield trauma, in which spinal cord injury was deemed: “An ailment not to be treated” Axon transection in traumatic nerve injury Modified from Brittis and Flanagan, Neuron (2001) 30, 11 Even after a microcrush injury, nerve axons fail to regenerate after injury optic nerve microcrush: Axon retraction Regeneration failure 24 h post-injury 2 wks post-injury 100100 µm µm Selles-Navarro, et al. (2001) Exp. Neurol. 167, 282-289 • The peripheral nervous system (PNS) is more permissive for axon regeneration than the central nervous system (CNS). • When PNS nerve sheath is grafted into a CNS injury, some CNS axons regenerate through the graft Adult Rat CNS axon regeneration David & Aguayo (1981) Science 214, 931-933 In vitro, superior cervical ganglion neurites extend on a surface coated without myelin (P) or on PNS myelin (PR), but not on a surface coated with CNS myelin (CR) Caroni & Schwab (1988) J Cell Biol 106, 1281-1288 Axon transection in traumatic nerve injury Modified from Brittis and Flanagan, Neuron (2001) 30, 11 Multiple axon regeneration inhibitors (ARI’s) accumulate at the site of a CNS injury • Myelin-associated glycoprotein (MAG) – on residual myelin • Nogo – on residual myelin • OMgp – on residual myelin • Chondroitin sulfate proteoglycan (CSPG) – on residual myelin and the astroglial scar Blocking one or more ARI may enhance axon regeneration after -

Chronic Traumatic Encephalopathy: the Dangers of Getting “Dinged” Shaheen E Lakhan1* and Annette Kirchgessner1,2
Lakhan and Kirchgessner SpringerPlus 2012, 1:2 http://www.springerplus.com/content/1/1/2 a SpringerOpen Journal REVIEW Open Access Chronic traumatic encephalopathy: the dangers of getting “dinged” Shaheen E Lakhan1* and Annette Kirchgessner1,2 Abstract Chronic traumatic encephalopathy (CTE) is a form of neurodegeneration that results from repetitive brain trauma. Not surprisingly, CTE has been linked to participation in contact sports such as boxing, hockey and American football. In American football getting “dinged” equates to moments of dizziness, confusion, or grogginess that can follow a blow to the head. There are approximately 100,000 to 300,000 concussive episodes occurring in the game of American football alone each year. It is believed that repetitive brain trauma, with or possibly without symptomatic concussion, sets off a cascade of events that result in neurodegenerative changes highlighted by accumulations of hyperphosphorylated tau and neuronal TAR DNA-binding protein-43 (TDP-43). Symptoms of CTE may begin years or decades later and include a progressive decline of memory, as well as depression, poor impulse control, suicidal behavior, and, eventually, dementia similar to Alzheimer’s disease. In some individuals, CTE is also associated with motor neuron disease similar to amyotrophic lateral sclerosis. Given the millions of athletes participating in contact sports that involve repetitive brain trauma, CTE represents an important public health issue. In this review, we discuss recent advances in understanding the etiology of CTE. It is now known that those instances of mild concussion or “dings” that we may have previously not noticed could very well be causing progressive neurodegenerative damage to a player’s brain. -

Chapter 18 CRANIAL NERVE INJURIES
Cranial Nerve Injuries Chapter 18 CRANIAL NERVE INJURIES † ‡ SCOTT B. ROOFE, MD, FACS*; CAROLINE M. KOLB, MD ; AND JARED SEIBERT, MD INTRODUCTION CRANIAL NERVE V CRANIAL NERVE VII Evaluation Imaging Electrodiagnostic Testing Eye Care Nerve Exploration Nerve Repair Interposition Grafting Intratemporal Injuries Facial Nerve Decompression LOWER CRANIAL NERVES, IX THROUGH XII CRANIAL NERVE IX CRANIAL NERVE X CRANIAL NERVE XI CRANIAL NERVE XII SUMMARY CASE PRESENTATIONS Case Study 18-1 Case Study 18-2 *Colonel, Medical Corps, US Army; Program Director, Otolaryngology Residency, Tripler Army Medical Center, 1 Jarrett White Road, Honolulu, Hawaii 96859-5000; Assistant Professor of Surgery, Uniformed Services University of the Health Sciences †Major, Medical Corps, US Army; Staff Otolaryngologist, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, Virginia 20112 ‡Captain, Medical Corps, US Army; Otolaryngology Resident, Tripler Army Medical Center, 1 Jarrett White Road, Honolulu, Hawaii 96859-5000 213 Otolaryngology/Head and Neck Combat Casualty Care INTRODUCTION Injuries to the head and neck rank as some of the cal to maximizing the outcomes for these patients. most common injuries suffered in the current combat The otolaryngologist clearly has a vital role in the environment. These injuries often lead to extensive management of nearly all cranial nerve injuries. bony and soft tissue disruption, which places the cra- However, in the combat environment, this specialty nial nerves at increased risk for damage. Functional generally focuses on deficits arising from cranial deficits of any of these nerves can be devastating to nerves VII and VIII as well as the lower cranial nerves, both appearance and function. Due to the complex IX through XII. -

Peripheral Nerve Regeneration and Muscle Reinnervation
International Journal of Molecular Sciences Review Peripheral Nerve Regeneration and Muscle Reinnervation Tessa Gordon Department of Surgery, University of Toronto, Division of Plastic Reconstructive Surgery, 06.9706 Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected]; Tel.: +1-(416)-813-7654 (ext. 328443) or +1-647-678-1314; Fax: +1-(416)-813-6637 Received: 19 October 2020; Accepted: 10 November 2020; Published: 17 November 2020 Abstract: Injured peripheral nerves but not central nerves have the capacity to regenerate and reinnervate their target organs. After the two most severe peripheral nerve injuries of six types, crush and transection injuries, nerve fibers distal to the injury site undergo Wallerian degeneration. The denervated Schwann cells (SCs) proliferate, elongate and line the endoneurial tubes to guide and support regenerating axons. The axons emerge from the stump of the viable nerve attached to the neuronal soma. The SCs downregulate myelin-associated genes and concurrently, upregulate growth-associated genes that include neurotrophic factors as do the injured neurons. However, the gene expression is transient and progressively fails to support axon regeneration within the SC-containing endoneurial tubes. Moreover, despite some preference of regenerating motor and sensory axons to “find” their appropriate pathways, the axons fail to enter their original endoneurial tubes and to reinnervate original target organs, obstacles to functional recovery that confront nerve surgeons. Several surgical manipulations in clinical use, including nerve and tendon transfers, the potential for brief low-frequency electrical stimulation proximal to nerve repair, and local FK506 application to accelerate axon outgrowth, are encouraging as is the continuing research to elucidate the molecular basis of nerve regeneration. -

Reanimating Hand Function After Spinal Cord Injury Using Nerve
rehabilitation a r t i c l e Mary Galea, AM FAHMS, B App Sc (Physio), BA, PhD is a Professorial Fellow at the Department of Medicine, University of Melbourne. Reanimating hand She is a Physiotherapist and Neuroscientist whose research programme includes both laboratory-based and clinical function after spinal projects with the overall theme of understanding the control of voluntary movement by the brain, and factors that promote recovery cord injury using following nervous system injury. Aurora Messina, BSc (Hons), PhD is a Senior Research Fellow at nerve transfer surgery the Department of Medicine and the Peter Doherty Institute, University of Melbourne, Australia. She is a Key Take Home Messages Age at injury, and injuries caused by morphologist with an interest 2 in the fields of nerve injury • Loss of hand and arm function is a falls have increased over time. Over half and regeneration, and tissue devastating consequence of cervical of the injuries affect the cervical spinal engineering. spinal cord injury cord,1 leading to tetraplegia, that is, some • Surgical approaches to reconstruct degree of paralysis in all four limbs as Bridget Hill, MCSP, arm and hand function in people with well as the trunk. In tetraplegia, the Postgrad Dip (Musc), PhD tetraplegia include tendon transfer degree of impairment of the upper limb, is a Physiotherapist and Early and nerve transfer surgery including the hand, will vary depending Career Researcher having • Nerve transfer surgery can be used on the level and completeness of injury. been awarded her PhD in in combination with tendon transfer Loss of function is greater the higher 2017. -

How Is the Brain Organized?
p CHAPTER 2 How Is the Brain Organized? An Overview of Brain Structure The Functional Organization Brain Terminology of the Brain The Brain’s Surface Features Principle 1: The Sequence of Brain Processing The Brain’s Internal Features Is “In Integrate Out” Microscopic Inspection: Cells and Fibers Principle 2: Sensory and Motor Divisions Exist Focus on Disorders: Meningitis and Throughout the Nervous System Encephalitis Principle 3: The Brain’s Circuits Are Crossed Focus on Disorders: Stroke Principle 4: The Brain Is Both Symmetrical and Asymmetrical Principle 5: The Nervous System Works A Closer Look at Neuroanatomy Through Excitation and Inhibition The Cranial Nervous System Principle 6: The Central Nervous System Has The Spinal Nervous System Multiple Levels of Function The Internal Nervous System Principle 7: Brain Systems Are Organized Both Focus on Disorders: Magendie, Bell, and Bell’s Hierarchically and in Parallel Palsy Principle 8: Functions in the Brain Are Both Localized and Distributed A. Klehr / Stone Images Micrograph: Carolina Biological Supply Co. / Phototake 36 I p hen buying a new car, people first inspect the In many ways, examining a brain for the first time is outside carefully, admiring the flawless finish similar to looking under the hood of a car. We have a vague W and perhaps even kicking the tires. Then they sense of what the brain does but no sense of how the parts open the hood and examine the engine, the part of the car that we see accomplish these tasks. We may not even be responsible for most of its behavior—and misbehavior. able to identify many of the parts. -
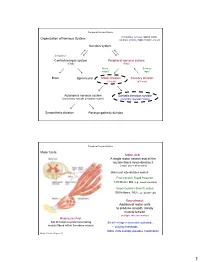
Nervous System Central Nervous System Peripheral Nervous System
Peripheral Nervous System Involuntary reflexes (spinal cord); Organization of Nervous System: voluntary actions (higher brain centers) Nervous system Integration Central nervous system Peripheral nervous system (CNS) (PNS) Motor Sensory output input Brain Spinal cord Motor division Sensory division (efferent) (afferent) Autonomic nervous system Somatic nervous system (involuntary; smooth & cardiac muscle) (voluntary; skeletal muscle) Sympathetic division Parasympathetic division Peripheral Nervous System Motor Units: Motor Unit: A single motor neuron and all the muscle fibers innervated by it (motor unit = all-or-none) Motor unit size dictates control: Fine Control / Rapid Reaction: 1-10 fibers / MU (e.g., ocular muscles) Gross Control / Slow Reaction: 1000’s fibers / MU (e.g., quadriceps) Recruitment: Addition of motor units to produce smooth, steady muscle tension (multiple fiber summation) Motoneuron Pool: Set of motor neurons innervating Small large motor units activated… muscle fibers within the same muscle • Varying thresholds Motor units overlap; provides coordination Marieb & Hoehn – Figure 9.13 1 Peripheral Nervous System Types of Motor Neurons: 1) Alpha () motor neurons: • Give rise to large Type A alpha (A) motor nerve fibers (~ 14 µm diameter) • Innervate extrafusal skeletal muscle fibers (generate force) 2) Gamma () motor neurons: • Give rise to small Type A gamma (Aγ) motor nerve fibers (~ 5 µm diameter) • Innervate intrafusal muscle fibers (small, specialized fibers – muscle spindle) What is the length of the muscle? Proper