Monitoring Study on Acid Rain in Kanagawa Prefecture, Central Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Yokohama Reinventing the Future of a City Competitive Cities Knowledge Base Tokyo Development Learning Center
COMPETITIVE CITIES FOR JOBS AND GROWTH CASE STUDY Public Disclosure Authorized YOKOHAMA REINVENTING THE FUTURE OF A CITY COMPETITIVE CITIES KNOWLEDGE BASE TOKYO DEVELOPMENT LEARNING CENTER October 2017 Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized © 2017 The World Bank Group 1818 H Street NW Washington, DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org All rights reserved. This volume is a product of the staff of the World Bank Group. The World Bank Group refers to the member institutions of the World Bank Group: The World Bank (International Bank for Reconstruction and Development); International Finance Corporation (IFC); and Multilater- al Investment Guarantee Agency (MIGA), which are separate and distinct legal entities each organized under its respective Articles of Agreement. We encourage use for educational and non-commercial purposes. The findings, interpretations, and conclusions expressed in this volume do not necessarily reflect the views of the Directors or Executive Directors of the respective institutions of the World Bank Group or the governments they represent. The World Bank Group does not guaran- tee the accuracy of the data included in this work. Rights and Permissions This work is a product of the staff of the World bank with external contributions. The findings, interpretations, and conclusions expressed in this work do not necessarily reflect the views of the World Bank, its Board of Executive Directors, or the governments they represent. Nothing herein shall constitute or be considered to be a limitation upon or waive of the privileges and immunities of the World Bank, all of which are specifically reserved. Contact: World Bank Group Social, Urban, Rural and Resilience Global Practice Tokyo Development Learning Center (TDLC) Program Fukoku Seimei Bldg. -
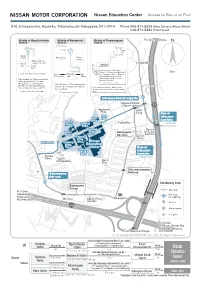
Map(Access by Train/Bus)
Nissan Education Center Access by Bus or on Foot 910, Ichisawa-cho, Asahi-ku, Yokohama-shi, Kanagawa 241-0014 Phone 045-371-5523 (Area General Affairs Office) 045-371-5334 (Front desk) Vicinity of Higashi-totsuka Vicinity of Wadamachi Vicinity of Futamatagawa ForFor Shin-yokohamaShin-yokohama N Station Station Station Drugstore 2 1 North Exit 2 1 Ticket gate South Exit Book store BookstoreBookstore Super market Stand-and-eat soba noodle bar LAWSONLAWSON West 2 upstairs Exit Ticket gate 1 Take the bus for "Sakonyama Dai-go", or "Sakonyama Dai-roku", 50m East Exit (For Aurora City) ForFor TicketTicket ForFor or "Tsurugamine Eki", or "Higashi- YokohamaYokohama gategate EbinaEbina totsuka Eki Nishi-guchi" at the Take the bus for "Sakonyama keiyu Futamatagawa Station South Exit bus stop No.1. Futamatagawa Eki","Ichisawa * Leaves every 10 min. Shogakkou", or "Sakonyama Take the bus for "Shin-sakuragaoka Dai-ichi" at the Higashi-Totsuka Danchi" at the Wadamachi Station Station West Exit bus stop No.2. Depending on the time, traffic can be bus stop No.1. heavily congested. Please allow enough * Leaves every 20 to 30 min. * Leaves every 20 min. time. Ichisawa Danchi Iriguchi IIchisawachisawa DDanchianchi HHigashi-gawaigashi-gawa 17 AApartmentpartment HHACAC BBldgldg . DDrugrug Kan-ni EastEast Ichisawa- EastEast gategate Bldg.Bldg. kamicho NorthNorth FamilyMartFamilyMart Bldg.Bldg. CentralCentral (Inbound)(Inbound) Bldg.Bldg. TrainingTraining Kan-niKan-ni WestWest Bldg.Bldg. SakonyamaSakonyama Ichisawa-Ichisawa- Bldg.Bldg. No.3No.3 Dai-rokuDai-roku kamichokamicho TrainingTraining Dai-ichiDai-ichi Bldg.Bldg. ParkPark MainMain gategate TTrainingraining No.2No.2 NissanNissan Bldg.Bldg. EEducationducation NNo.1o.1 CCenterenter Kan-ni TrainingTraining Bldg.Bldg. -

10. Sources and Useful Links
10. Sources and useful links Keio and SFC ・Keio University Jyukusei website : http://www.gakuji.keio.ac.jp/index.html ・SFC- Housing Information for International Students: http://www.gakuji.keio.ac.jp/en/sfc/sl/housing.html ・Keio University International Center – Housing for International Students: http://www.ic.keio.ac.jp/en/life/housing/ryu_boshu.html Housing Information ・Housemate – Shonandai branch: http://www.housemate-navi.jp/shop/59 ・Mini Mini - Shonandai branch: http://minimini.jp/shop/10016/shonandai/index.html ・Leopalace 21: For International Students: http://en.leopalace21.com/students/ ・ABLE: For International Students: http://www.able.co.jp/international/ ・Kyoritsu Maintenance: http://www.gakuseikaikan.com/international/english/index.html http://www.kif-org.com/placehall/e04.html ・Tokyo Sharehouse: http://tokyosharehouse.com/eng ・Oakhouse: http://www.oakhouse.jp/eng/ ・Sakura House: http://www.sakura-house.com/en ・Sharetomo Program http://www.sharetomo.jp/ Visa and Administration ・Immigration Bureau of Japan: http://www.immi-moj.go.jp/newimmiact_1/en/index.html ・Fujisawa City: https://www.city.fujisawa.kanagawa.jp/jinkendanjyo/gaikokugo/english/index.html ・Yamato City: http://www.city.yamato.lg.jp.e.gg.hp.transer.com/ http://www.city.yamato.lg.jp/web/content/000143006.pdf ・Chigasaki City: http://www.city.chigasaki.kanagawa.jp.e.ox.hp.transer.com/about/1010015.html http://www.city.chigasaki.kanagawa.jp.e.ox.hp.transer.com/kankyo/ 89 ・Yokohama City: http://www.city.yokohama.lg.jp/lang/en/ http://www.city.yokohama.lg.jp/lang/en/5-3-1.html -
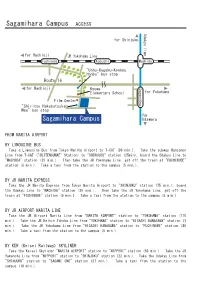
Sagamihara Campus ACCESS Odakyu Line
Sagamihara Campus ACCESS Odakyu line for Shinjuku for Hachioji JR Yokohama Line Fuchinobe Kobuchi Machida "Uchu-Kagaku-Kenkyu Honbu" bus stop Route 16 for Hachioji Kyowa SagamiOno Elementary School for Yokohama Film Center "Shiritsu Hakubutsukan Mae" bus stop for Sagamihara Campus Odawara FROM NARITA AIRPORT BY LIMOUSINE BUS Take a Limousine Bus from Tokyo Narita Airport to T-CAT (90 min.). Take the subway Hanzomon Line from T-CAT ("SUITENGUMAE" Station) to "SHINJUKU" station (25min), board the Odakyu Line to "MACHIDA" station (35 min.). Then take the JR Yokohama Line, get off the train at "FUCHINOBE" station (6 min.). Take a taxi from the station to the campus (5 min.). BY JR NARITA EXPRESS Take the JR Narita Express from Tokyo Narita Airport to "SHINJUKU" station (75 min.), board the Odakyu Line to "MACHIDA" station (35 min.). Then take the JR Yokohama Line, get off the train at "FUCHINOBE" station (6 min.). Take a taxi from the station to the campus (5 min.). BY JR AIRPORT NARITA LINE Take the JR Airport Narita Line from "NARITA AIRPORT" station to "YOKOHAMA" station (110 min.). Take the JR Keihin Tohoku Line from "YOKOHAMA" station to "HIGASHI KANAGAWA" station (3 min.). Take the JR Yokohama Line from "HIGASHI KANAGAWA" station to "FUCHINOBE" station (40 min.). Take a taxi from the station to the campus (5 min.). BY KER (Keisei Railway) SKYLINER Take the Keisei Skyliner "NARITA AIRPORT" station to "NIPPORI" station (50 min.). Take the JR Yamanote Line from "NIPPORI" station to "SHINJUKU" station (22 min.). Take the Odakyu Line from "SHINJUKU" station to "SAGAMI ONO" station (37 min.). -

Why Kanagawa? Business Environment & Investment Incentives
Why Kanagawa? Business Environment & Investment Incentives Investment Environment International Business Group Investment Promotion and International Business Division Industry Department Industry and Labor Bureau Kanagawa Prefectural Government Leading the way in adopting Western culture, Japan’s modernization began here. 1 Nihon-Odori, Naka-ku, Yokohama, Kanagawa Located the ideal distance from Tokyo, Kanagawa retains its own unique appeal. 231-8588 Japan Rich natural environments from the shores of Shonan to the mountains of Hakone. Rail and highway networks encompassing the Tokyo Metro Area. Tel: +81-45-210-5565 http://www.pref.kanagawa.jp/div/0612/ And now, with the new investment incentive program, “Select Kanagawa 100,” KANAGAWA will shine even brighter! June 2016 Welcome to Kanagawa Prefecture Forming a mega-market with the bordering capital city of Tokyo, Kanagawa Prefecture itself boasts a population exceeding 9.1 million. It is home to a high concentration of R&D facilities and offices of multinational corporations, as well as many small- and medium-sized businesses with exceptional technological capabilities. In addition to an expansive and well-developed highway and rail network, Kanagawa also offers extensive urban facilities and industrial infrastructure, including the international trading ports of Yokohama, Kawasaki, and Yokosuka, with Haneda International Airport located nearby. Kanagawa is also blessed with a lush natural environment of verdant mountains and picturesque coastlines, and features numerous sightseeing spots which encapsulate all of Japan’s charms. These include the international port city of Yokohama, the historic and culturally vibrant ancient samurai capital of Kamakura, and Hakone, the international tourist destination known for its hot springs and magnificent scenery of Mt. -

Teamlab Future Park
Kanagawa sightseeing charm cration conference Shonan <Mag-cul・Amusement> Hiratsuka City Think with your body, Tourist Attraction No. and grow through collaborative creation. 1901 teamLab Future Park Get creative by moving around and drawing in this digital art space. Color your favorite sea creatures or buildings with crayons and see it come to life in Explanation of the aquarium or in the town. The space has nine artworks including "Sliding Tourist Attraction through the Fruit Field", "Sketch Town", and "Sketch Aquarium." teamLab has created a space for family fun in ""Lalaport Shonan Hiratsuka"" selling point alongside the commercial center's restaurants and shopping areas. This permanent teamLab space is one of only four in all of Japan. Address LaLaport SHONAN HIRATSUKA 3F 700-17 Amanuma Hiratsuka-shi Kanagawa-ken Opening Hours 10:00-18:00 (Last admission 17:30) Corresponds with the days of operation of Lalaport Shounan Hiratsuka. Availability of Parking Use Lalaport Shonan-Hiratsuka Car Park (3,000 spaces) URL https://futurepark.teamlab.art/event/lalaporthiratsuka Recommended Season All year Access Group/Individual Mark Individual ①From bus stop 5 of the north exit of Hiratsuka Station on JR Tokaido Line take a Kanagawa Target Regions Europe, North America, Oceania, Asia Chuo Bus bound for Hiratukaeki-Kitaguchi (Hira 11 Route) for about 5 minutes. Get off at the Lalaport Shonan-Hiratsuka bus stop. ②12 mins. walk from Hiratsuka Station (JR Tokaido Line) Specific Model Route Details Individual JR Tokaido Line [Yokohama Station] +++(30 -

YOKOHAMA and KOBE, JAPAN
YOKOHAMA and KOBE, JAPAN Arrive Yokohama: 0800 Sunday, January 27 Onboard Yokohama: 2100 Monday, January 28 Arrive Kobe: 0800 Wednesday, January 30 Onboard Kobe: 1800 Thursday, January 31 Brief Overview: The "Land of the Rising Sun" is a country where the past meets the future. Japanese culture stretches back millennia, yet has created some of the latest modern technology and trends. Japan is a study in contrasts and contradictions; in the middle of a modern skyscraper you might discover a sliding wooden door which leads to a traditional chamber with tatami mats, calligraphy, and tea ceremony. These juxtapositions mean you may often be surprised and rarely bored by your travels in Japan. Voyagers will have the opportunity to experience Japanese hospitality first-hand by participating in a formal tea ceremony, visiting with a family in their home in Yokohama or staying overnight at a traditional ryokan. Japan has one of the world's best transport systems, which makes getting around convenient, especially by train. It should be noted, however, that travel in Japan is much more expensive when compared to other Asian countries. Japan is famous for its gardens, known for its unique aesthetics both in landscape gardens and Zen rock/sand gardens. Rock and sand gardens can typically be found in temples, specifically those of Zen Buddhism. Buddhist and Shinto sites are among the most common religious sites, sure to leave one in awe. From Yokohama: Nature lovers will bask in the splendor of Japan’s iconic Mount Fuji and the Silver Frost Festival. Kamakura and Tokyo are also nearby and offer opportunities to explore Zen temples and be led in meditation by Zen monks. -

Program 2017-2018
神奈川大学 日本語・日本文化プログラム PROGRAM 2017-2018 SUMMER : JUNE 30[ FRI]- AUGUST 4[ FRI], 2017 APPLICATION PERIOD JANUARY 10[ TUE]- MARCH 31[ FRI], 2017 WINTER : JANUARY 12[ FRI]- FEBRUARY 16[ FRI], 2018 APPLICATION PERIOD JULY 4[ TUE]-SEPTEMBER 29[ FRI], 20 17 International Exchange Kanagawa University Kanagawa University seeks to prepare its students Kanagawa University is committed to creating a more for active involvement in the international arena. attractive academic environment in every dimension This has been an objective ever since the university’s of campus life, from education and research to social founding in 1928, and is now guided by a fundamental contribution. With its Yokohama and Shonan Hiratsuka policy on international exchange. Our efforts to Campuses, Kanagawa University is a comprehensive further the cause of international exchange involve university with seven undergraduate faculties consisting the conclusion of academic exchange agreements of 20 departments and two programs, and nine with universities overseas, as well as the expansion graduate schools offering 16 courses. Students can of short-term training programs with universities choose to major in any of a large number of disciplines beyond these formal exchange agreements. in the humanities and sciences. Yokohama campus is a functional and beautiful campus, in a location with convenient access to the Yokohama station area, Overview of Yokohama and major sightseeing spots Minatomirai area and Shibuya, Tokyo. Yokohama is located in the center of Japan, along the coastline of the Pacific Ocean, and is one of the 20 Japanese Government-designated cities. The average temperature is 32°C with high humidity in the summers and 5.9°C with low humidity in the winters. -

CV in English
Sarah Brayer sarahbrayer.com [email protected] ________________ born: Rochester, New York lives: Kyoto, Japan and New York since 1980 Solo Exhibitions 2019 Indra’s Cosmic Net, Daitokuji Studio, Kyoto 2018 Kyoto Passages, The Ren Brown Collection, Bodega Bay, California The Red Thread, Daitokuji Studio, Kyoto 2016 Celestial Threads, Daitokuji Studio, Kyoto ArtHamptons, The Tolman Collection: New York 2015 Luminosity, Hanga Ten, London, England Luminosity, Daitokuji Studio, Kyoto 2014 Between Two Worlds: Poured Paperworks by Sarah Brayer, Castellani Art Museum, Niagara University. catalog In the Moment, Gallery Bonten, Shimonoseki, Japan 2013 Tiger’s Eye, The Verne Collection, Cleveland, Ohio Cloud Garden Paperworks, The Ren Brown Collection, Bodega Bay, California 2012 Luminosity: Night Paperworks, Reike Studio, Santa Fe, New Mexico Light and Energy, Gallery Bonten, Shimonoseki, Japan Recent Works by Sarah Brayer, The Tolman Collection: New York 2011 East Meets West, The Tolman Collection, New York, NY New Works in Washi & Glass, Gallery Shinmonzen, Kyoto The Schoolhouse Gallery, Mutianyu, Beijing, China Gallery Bonten, Shimonoseki, Japan 2010 Luminosity: Night Paperworks, Kamigamo Studio, Kyoto Mythos, The Ren Brown Collection, Bodega Bay, California Art in June, Rochester, New York 30 Years of Art in Kyoto: Sarah Brayer Studio, Kyoto 2007 The Ren Brown Collection, Bodega Bay Gallery Bonten, Shimonoseki, Japan Round the Horn, Nantucket Art in June, Rochester, New York 2006 Whisper to the Moon, Iwakura Kukan, Kyoto Art in June, Rochester, -

Yokohama Rulebook
●Trade Good components ・30 Copper tokens ・30 Raw Silk tokens ・40 Tea tokens ・40 Seafood tokens よこはま しんしょう でん ●23 Imported Goods tokens ●Money 2-4 players / 90 minutes / ages 12 and above ・52 1-Yen tokens 1 ・16 3-Yen tokens 3 Setting of the Game It is the beginning of the Meiji era. Once just a sleepy ●1 Station token 1 fishing village, the opening of foreign trade in Yokoha- ma and the decline of Edo led Yokohama to become the ●10 Foreign Mercenaries premier hub of trade in all of Japan. During this period, Japanese products such as copper and raw silk were Front Back established in Yokohama to be exported to foreign countries. In addition, foreign technology and culture 3 English were incorporated into Japan, and modernization slow- 2 American ly swept over the streets of the city. Behind all of these 2 French incredible developments were the capable merchants of 2 German Yokohama. 1 Dutch Overview of the Game ●20 5-Power tokens Each player is a merchant of Yokohama, vying to gain fame through successful business ventures. Front Back There are a number of ways to gain fame, such as dis- playing trade goods ordered by foreign traders, learning 18 Area Boards foreign technology, and developing the city by building ● shophouses and trading houses. In order to fulfill these Front goals, players will need to roam the streets of Yokoha- Area CategoryArea Rule ma, gaining its various benefits. In addition, it may be a Area Name 教会 Church Space for Location card good idea to seek the cooperation of foreign mercenar- 2 Common ies. -

History of City Planning in the City of Yokohama
History of City Planning in the City of Yokohama City Planning Division, Planning Department, Housing & Architecture Bureau, City of Yokohama 1. Overview of the City of Yokohama (1) Location/geographical features Yokohama is located in eastern Kanagawa Prefecture at 139° 27’ 53” to 139° 43’ 31” East longitude and 35° 18’ 45” to 35° 35’ 34” North latitude. It faces Tokyo Bay to the east and the cities of Yamato, Fujisawa, and Machida (Tokyo) to the west. The city of Kawasaki lies to the north, and the cities of Kamakura, Zushi, and Yokosuka are to the south. Yokohama encompasses the largest area of all municipalities in the prefecture and is the prefectural capital. There are also rolling hills running north-south in the city’s center. In the north is the southernmost end of Tama Hills, and in the south is the northernmost end of Miura Hills that extends to the Miura Peninsula. A flat tableland stretches east-west in the hills, while narrow terraces are partially formed along the rivers running through the tableland and hills. Furthermore, valley plains are found in the river areas and coastal lowland on the coastal areas. Reclaimed land has been constructed along the coast so that the shoreline is almost entirely modified into manmade topography. (2) Municipal area/population trends The municipality was formed in 1889 and established the City of Yokohama. Thereafter, the municipal area was expanded, a ward system enforced, and new wards created, resulting in the current 18 wards (administrative divisions) and an area of 435.43km2. Although the population considerably declined after WWII, it increased by nearly 100,000 each year during the period of high economic growth. -

Data Book 2011
Financial Highlights THE YOKOHAMA RUBBER CO., LTD. (Millions of yen) FY2007 FY2008 FY2009 FY2010 FY2011 Net sales 497,396 551,431 517,262 466,358 519,742 DATA BOOK 2011 Tires 372,708 419,834 399,728 367,571 411,574 MB 124,687 131,596 117,534 98,787 108,167 Industrial Products — — — 73,967 83,835 Others — — — 24,819 24,332 Operating income 21,069 33,118 12,808 21,454 29,490 Net income (loss) 16,363 21,060 (5,654) 11,486 13,923 Total assets 536,322 526,191 473,376 466,973 478,915 Net income (loss) per share (Yen) 48.79 62.81 (16.87) 34.27 41.55 Net assets per share (Yen) 542.10 525.96 417.45 475.26 489.27 Equity ratio (%) 33.9 33.5 29.6 34.1 34.2 Corporate Data Capital expenditure 40,638 27,292 43,340 17,471 24,944 Number of consolidated Company Name The Yokohama Rubber Company, Limited 157 155 156 127 120 subsidiaries Established October 13, 1917 Number of employees 15,423 16,099 16,772 17,566 18,465 Paid-in Capital ¥38,909 million (As of March 31, 2011) Net Sales ¥519,742 million (Consolidated basis, year ended March 31, 2011) Net sales Operating income Fiscal Year-end December 31 (Change in fi scal year-end) (Millions of yen)Tires MB (Millions of yen) 600,000 40,000 Chairman and CEO and Tadanobu Nagumo 551,431 517,262 519,742 Representative Director 497,396 33,118 466,358 Head Offi ce 36-11, Shimbashi 5-chome, Minato-ku, Tokyo 105-8685, Japan 131,596 108,167 29,490 117,534 30,000 Factory and Plants Hiratsuka, Mie, Mishima, Shinshiro, Ibaraki, Onomichi, Nagano, 124,687 98,787 400,000 Shinshiro-Minami, Hiratsuka-East 21,069 21,454 Tire Test Courses Daigo Proving-ground and Research Center (D-PARC) / Ibaraki 20,000 Takasu Motoring and Researching Yard (T MARY) / Hokkaido 419,834 411,574 372,708 399,728 367,571 12,808 TIRE TEST CENTER OF ASIA / Thailand 200,000 Overseas Subsidiaries Yokohama Tire Corporation (U.S.) 10,000 Yokohama Tire (Canada) Inc.