Black Match” …………………………………………… P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From a Technical Standpoint, What Is Firework Flash Powder?
This article originally appeared in Fireworks Business, No. 246, 2004. From a Technical Standpoint, What Is Firework Flash Powder? K. L. Kosanke and L. Weinman perature attainable in pyrotechnic reactions, only Introduction solids and liquids incandesce, gases do not. As a In a recently published article on the regulatory practical matter, metal oxides are the only pyro- definitions of firework flash powder[1] it was con- technic reaction products that are not gaseous at cluded that none of those definitions provided suf- high temperature. Thus metal fuels need to be pre- ficient information to objectively establish wheth- sent in substantial quantity in flash powders. See er or not a pyrotechnic composition is a flash Table 1, which is a list of the boiling point of powder. That is to say, those definitions are all some of the pyrotechnic reaction products. The subjective to the extent that they depend on the table has somewhat arbitrarily been divided into intended use of the composition and none provide species with boiling points above and below a quantifiable measure that can be used to deter- 2500 °C. Note that the oxides of zirconium, mag- mine whether a particular pyrotechnic composi- nesium, aluminum and titanium top the list. tion is a flash powder. The purpose of the present article is to suggest a general approach that might Table 1. Boiling Point of Some Pyrotechnic be used as the basis for producing a quantitative [2] definition of flash powder. Reaction Products. The reason such an objectively quantifiable Reaction Product Boiling Point (°C) definition is needed is that – from both a regulato- ZrO2 ≈ 5000 ry and safety standpoint – flash powders are treat- MgO 3600 ed differently than other pyrotechnic composi- Al2O3 2980 tions. -

ENCYCLOPEDIA of CHEMISTRY & EXPLOSIVES MATERIALS Abelite
ENCYCLOPEDIA OF CHEMISTRY & EXPLOSIVES MATERIALS A Abelite An explosive, composed mainly of ammonium nitrate and trinitrotoluene. Absolute Zero The least possible temperature for all substances. Generally accepted as -273.15ÝC AC Alternating current. Acceptance Quality Level (AQL) A nominal value expressed in terms of percentage defective per hundred units, by which a group of sampling plans is identified. The sampling plans so identified have a high probability of accepting lots containing material with a process average not greater than the designed value of the AQL. Acetin [CH3COOC3H5(OH)2] also known as glyceryl monoacetate, a colourless hydroscopic liquid. Used as an intermediate for various explosives, and a solvent for various dyes. Acetone [CH3COCH3] colourless, flammable liquid. Acetone is widely used in industry as a solvent for many organic substances. It is used in making synthetic Resins and fillers, smokeless powders, and many other organic compounds. Boiling Point 56ÝC. Useful solvent for acetylene, also known as the simplest saturated ketone. Acetylene or ethyne, a colourless gas and the simplest alkyne Hydrocarbon. Explosive on contact with air, it is stored dissolved under pressure in Acetone. It is used to make neoprene rubber, plastics, and resins. The oxyacetylene torch mixes and burns oxygen and acetylene to produce a very hot flame-as high as 3480ÝC (6300ÝF)-that can cut steel and weld iron and other metals. Produced by the action of wateron calcium carbide and catalytically from naphtha. Acetylide A carbide formed by bubbling acetylene through a metallic salt solution, eg cuprous acetylide, Cu2C2. These are violently explosive compounds. Acid Any substance capable of giving up a proton; a substance that ionizes in solution to give the positive ion of the solvent; a solution with a pH measurement less than 7. -

WPA Newsletter, Volume 25, Issue 1
WPA Newsletter, Volume 25, Issue 1 Table of Contents Corporate Members 4 Editorials 5 Passages 8 Winter Blast Round-Up 12 Burro Races 16 Guest photographer Kelly 18 Maker Faire 27 Cover Picture - “BANG! BANG!” News from Here and There 28 Photo by Kelly Dreller DO IT announcement 31 Elected Officers of the WPA New Rocket Policy 33 President: Steve Wilson Moapa 33 Vice President: Greg Dandurand Blackfinger’s Insert Workshop 34 VP Publications: Pete Hand Treasurer: Richard Haase Secretary: Dennis Miele THE SMALL PRINT The Western Pyrotechnic Association, Inc., also known as the WPA, is a non-profit group of fireworks professionals and their apprentices. This newsletter is a vehicle for their exchange of information in this craft and the right to publish this information is guaranteed by the Constitution of the United States of America. Nonetheless, readers are urged to learn and obey all laws and regulations of all federal, state, and local jurisdictions and of their agencies and representatives. Some information herein may contain incom- plete descriptions of fireworks techniques based on the experience of its author(s) in a controlled environment with circumstances, and conditions different from the reader. Readers must form their own opinion as to the application of this information. This information is considered documentary in nature and no opinion is given as to its suitability or use. No warranties are made either expressed or implied, including but not limited to warranties of the accuracy of the information herein. The WPA is not responsible for the opinions of authors or mistakes in printing. All information is intended solely for viewing by members of the Western Pyrotechnic Association, Inc. -
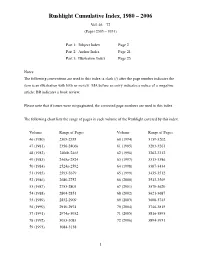
Rushlight Index 1980-2006
Rushlight Cumulative Index, 1980 – 2006 Vol. 46 – 72 (Pages 2305 – 3951) Part 1: Subject Index Page 2 Part 2: Author Index Page 21 Part 3: Illustration Index Page 25 Notes: The following conventions are used in this index: a slash (/) after the page number indicates the item is an illustration with little or no text. MA before an entry indicates a notice of a magazine article; BR indicates a book review. Please note that if issues were mispaginated, the corrected page numbers are used in this index. The following chart lists the range of pages in each volume of the Rushlight covered by this index. Volume Range of Pages Volume Range of Pages 46 (1980) 2305-2355 60 (1994) 3139-3202 47 (1981) 2356-2406a 61 (1995) 3203-3261 48 (1982) 2406b-2465 62 (1996) 3262-3312 49 (1983) 2465a-2524 63 (1997) 3313-3386 50 (1984) 2524a-2592 64 (1998) 3387-3434 51 (1985) 2593-2679 65 (1999) 3435-3512 52 (1986) 2680-2752 66 (2000) 3513-3569 53 (1987) 2753-2803 67 (2001) 3570-3620 54 (1988) 2804-2851 68 (2002) 3621-3687 55 (1989) 2852-2909 69 (2003) 3688-3745 56 (1990) 2910-2974 70 (2004) 3746-3815 57 (1991) 2974a-3032 71 (2005) 3816-3893 58 (1992) 3033-3083 72 (2006) 3894-3951 59 (1993) 3084-3138 1 Rushlight Subject Index Subject Page Andrews' burning fluid vapor lamps 3400-05 Abraham Gesner: Father of Kerosene 2543-47 Andrews patent vapor burner 3359/ Accessories for decorating lamps 2924 Andrews safety lamp, award refused 3774 Acetylene bicycle lamps, sandwich style 3071-79 Andrews, Solomon, 1831 gas generator 3401 Acetylene bicycle lamps, Solar 2993-3004 -

Safety Guidelines of the PGI Official Fireworks Safety Guidelines in Effect at PGI Conventions Pyrotechnics Guild International, Inc
Safety Guidelines of the PGI Official Fireworks Safety Guidelines in effect at PGI Conventions Pyrotechnics Guild International, Inc. 2019 TABLE OF CONTENTS 1. Authority and Scope . 2 2. Safety Committee . 2 3. General Requirements . 3 4. Class B/1.3G Testing . 6 5. Competition . 7 6. Public Display Area . 8 7. Class C/1.4G Sales . 19 8. Class C/1.4G Shooting . 21 9. Auction . 23 10. Assembly of Pyrotechnic Devices . 23 11. Educational Workshops and Demonstrations . 24 12. Trade Show Sales . 24-25 13. Storage . 25 14. Minimum Distance From Spectators . 26 15. Anvil Firing . 28 16. Manufacturing . 28 2019 Committee for Safety Guideline Revision John Steinberg, Chairman Paul Smith Carol Hostetter Jim Beardmore Ruth Newhouse Beardmore 1. AUTHORITY AND SCOPE 1. These guidelines shall be known as the Official Fireworks Safety Guidelines and be cited as such. They shall be referred to herein as “The Guidelines.” 2. Authority: These guidelines were adopted by the Pyrotechnics Guild International, Inc. (PGI) in July 1983; revised July 1987, revised July 1990 [to incorporate changes to NFPA 1123 (1990)], revised June 1994 [to cover the rapid growth of PGI membership]; revised in April 2000 ; revised in May 2001; revised in November of 2008; and revised again in the spring of 2019 and shall remain an official document of the PGI until amended or discontinued. As NFPA codes are continually revised, NFPA-1123, NFPA-1126, and NFPA-160 in their current editions should serve to guide future revisions of these Safety Guidelines. 3. Scope: These Guidelines apply to the handling, storage, sale, discharge or other use of all kinds of fireworks and pyrotechnic or explosive devices during any official PGI convention. -

Pyrotechnic Serpents
Edited by Jack & Dorothy Drewes American Fireworks News THE BEST OF AFN III Edited by Jack & Dorothy Drewes Copyright ©1995 by Rex E. & S. P., Inc. Published by American Fireworks News HC67 - Box 30 Dingmans Ferry, PA 18328 All rights reserved. ISBN 0-929931-11-4 Printed in The United States of America 1st printing, April, 1995. 2nd printing, March, 1996 3rd printing, March, 1998 Warning: This publication contains descriptions and pictures of fireworks. The information contained herein is based on the authors' experiences using specific tools and ingredients under specific conditions not necessarily described in the articles. No warranties are made, given or implied. Readers are cautioned that they must form their own opinion as to the application of any information contained herein. 2 CONTENTS BASICS, SMALL DEVICES DISPLAY GOODS & OPERATIONS Getting a Pyro Education 7 The Basic Technician, #1, 2, 3 62 Fireworks and Me 8 Unexplained Explosion & Probability Lightning & Thunder Fountain 9 Theory 66 Construction Techniques of %" Roman Pyro Emitting Device 67 Candle Using Round Stars 10 Fireworks on a Budget 68 Bigger & Better Breaks with Small Ball Vis-A-Vis Fountains 70 Shells 11 Neon Blue & Recumbent Lances 71 Designing Portfires 12 Molecular Sieves as Cores 72 Fun with Jumping Jacks 14 Lance Development 73 Tischfeuerwerk 15 Illumination Breaks & Shimmering Bike Wheel Pyro 16 Curtains 73 Ground Bloom Flower Wheel 16 Pyro Surprises 74 Easy Sun 18 Push Sticks Aid Low Breaks 75 Class C Repeaters 19 Eight Experiments in Non-Commercial Exploding -

M-80 (Explosive) - Wikipedia,Visited the Free Encyclopediaon 8/14/2015 Page 1 of 4
M-80 (explosive) - Wikipedia,visited the free encyclopediaon 8/14/2015 Page 1 of 4 M-80 (explosive) From Wikipedia, the free encyclopedia For other uses, see M80. M-80s are an American class of large firecrackers, sometimes called SALUTES.[1] The Simulator, Artillery, M-80 was originally made in the early 20th century by the U.S. military to simulate explosives or artillery fire;[2] later, M-80s were manufactured as fireworks. Traditionally, M-80s were made from a small cardboard 1 tube, often red, approximately 1 ⁄2 inches (3.8 cm) long 9 and ⁄16 inch (1.4 cm) inside diameter, with a fuse or wick coming out of the side; this type fuse is commonly known as cannon fuse or Visco fuse, after a company responsible for standardizing the product. The tubes M-80 Salute (BOOTLEG) often hold approximately 2½–3 grams of pyrotechnic flash powder; many sources state that an M-80 carries 3 grams of powder.[3] Contents ◾ 1 Legality ◾ 1.1 Canada ◾ 1.2 United States ◾ 2 Accidents ◾ 3 References ◾ 4 Further reading Legality Canada https://en.wikipedia.org/wiki/M-80_(explosive) 8/14/2015 M-80 (explosive) - Wikipedia,visited the free encyclopediaon 8/14/2015 Page 2 of 4 M-80s are not authorized under the law, thus making importation, possession, transportation, storage or manufacturing illegal in Canada.[4] Firecrackers, including the M-80, can be purchased from Native Reserves in Canada, as they have different governing laws. United States Due to property damages and bodily harm caused by M-80s, Class C fireworks—now known as consumer fireworks (class 1.4G), as opposed to display fireworks (which were Class B, and are now 1.3G)[5]— civilians require a license, issued by federal authorities, for pyrotechnic devices containing a charge in excess of 50 milligrams of pyrotechnic flash powder. -

2013 Fireworks Safety Standards Development Status Report
FIREWORKS SAFETY STANDARDS DEVELOPMENT PROJECT FY 2013 STATUS REPORT October 2013 For further information contact: Dr. Christopher J. Musto, Project Manager Directorate for Laboratory Sciences U.S. Consumer Product Safety Commission (301) 987-2090 [email protected] Dr. Andrew Lock Directorate for Laboratory Sciences U.S. Consumer Product Safety Commission (301) 987-2099 [email protected] This report was prepared by the CPSC staff, has not been reviewed or approved by, and may not necessarily reflect the views of, the Commission. EXECUTIVE SUMMARY The U.S Consumer Product Safety Commission (CPSC, the Commission) is evaluating the need to amend its regulations for fireworks devices. CPSC staff evaluated issues concerning the explosive power of aerial fireworks. Aerial fireworks devices intended to produce an audible effect are limited to 130 mg (2 grains) of pyrotechnic composition, according to 16 C.F.R. § 1500.17(a)(3). Because the regulation only limits the amount of pyrotechnic composition for devices intended to produce an audible effect, compliance determinations can be made only after a subjective determination of intent to produce an audible effect. In 2012, staff conducted testing to evaluate two different approaches to replacing the regulation limiting pyrotechnic composition of devices intended to produce an audible effect; staff considered potential alternative regulations, based on the explosive power of fireworks devices. The American Fireworks Standards Laboratory (AFSL) had previously developed a “Black Powder Equivalency Test.” CPSC staff investigated and found that this test can distinguish between the energetics of black powder, hybrid powders, and flash powder, but the test does not consider the particular construction of a shell. -

Washington State Patrol Crime Laboratory Division Materials
Washington State Patrol Crime Laboratory Division Materials Analysis EXPLOSIVES TRAINING MANUAL May 2018 Washington State Patrol Crime Laboratory Division Explosives Laboratory Training Manual Contents 1 INTRODUCTION ..................................................................................................................................... 7 1.1 PURPOSE AND SCOPE ................................................................................................................... 7 1.2 ORGANIZATION OF THE TRAINING MANUAL ................................................................................ 7 2 EXPLOSIVES ANALYSIS OVERVIEW ........................................................................................................ 9 2.1 OBJECTIVES ................................................................................................................................... 9 2.2 OVERVIEW ..................................................................................................................................... 9 2.2.1 Definitions ............................................................................................................................. 9 2.3 SUGGESTED READINGS ............................................................................................................... 11 2.3.1 Introduction ........................................................................................................................ 11 2.3.2 Scene .................................................................................................................................. -

Chapter 212-17 WAC FIREWORKS
Chapter 212-17 Chapter 212-17 WAC FIREWORKS WAC 212-17-235 Pyrotechnic operators—Responsibility. 212-17-240 Pyrotechnic operators—Observance of laws, rules and PART I—GENERAL regulations. 212-17-001 Title. PART VII—PUBLIC DISPLAY LICENSE 212-17-010 Purpose. 212-17-015 Scope. 212-17-245 Public displays of fireworks—General. 212-17-020 Authority. 212-17-250 Public displays of fireworks—Application, state license. 212-17-025 Definition—"Fireworks." 212-17-255 Public displays of fireworks—Type of license. 212-17-030 Definition and classification—"Trick and novelty 212-17-260 Public displays of fireworks—General licenses. devices." 212-17-270 Public displays of fireworks—Local permit, application 212-17-032 Definition and classification—"Articles pyrotechnic." for. 212-17-035 Definition and classification—"Consumer fireworks." 212-17-275 Public displays of fireworks—Investigation. 212-17-040 Definition and classification—"Display fireworks." 212-17-280 Public displays of fireworks—Permits may not be 212-17-042 Definition and classification—"Special effects." granted, when. 212-17-045 Definition and classification—"Agricultural and wild- 212-17-285 Public displays of fireworks—Spectators. life fireworks." 212-17-290 Public displays of fireworks—Pyrotechnic operators. 212-17-050 Firework device chemical content, construction. 212-17-055 Firework device, labeling. PART VIII—PUBLIC DISPLAYS 212-17-060 Public purchase of fireworks. 212-17-295 Public display—General. PART II—MANUFACTURER 212-17-300 Public display—Definitions. 212-17-305 Public display—Construction of shells. 212-17-065 Fireworks manufacturer—General. 212-17-310 Public display—Storage of shells. 212-17-070 Fireworks manufacturer—Licensing. -

APA STANDARD 87-1 Contents 1
APA STANDARD 87-1 Contents 1. INTRODUCTION..............................................................................................1 2. DEFINITIONS.....................................................................................................1 3. REQUIREMENTS FOR CONSUMER FIREWORKS, NOVELTIES AND THEATRICAL PYROTECHNICS .....................................................................4 3.1 Types of Consumer Fireworks.......................................................................5 3.2 Types of Novelties .........................................................................................8 3.4 Other Devices ................................................................................................9 3.6 Specific Requirements for Consumer Fireworks...........................................10 3.7 Prohibited Chemicals and Components.........................................................12 3.8 Requirements for Theatrical Pyrotechnics ....................................................13 3.9 Approval ........................................................................................................13 3.10 Marking and Labeling..................................................................................14 4. REQUIREMENTS FOR DISPLAY FIREWORKS DEVICES ..........................14 4.1 Types of Display Fireworks Devices.............................................................14 4.2 Construction of Aerial Shells.........................................................................15 4.3 Approval -

Part II EXPLOSIVES INCIDENTS ANALYSIS
If you have issues viewing or .accessing this file contact us at NCJRS.gov. lIP :. I , . •• , .. I , It • ~ • -. I .. • r • -. I , I "$: .; ~.. ,~--.~.~. " I I I I ,.. .~;- ""', ....... ~~-, ~,-- - --------~- .. ". ---------------- Cover: Top Photo: The results of an explosive device that detonated beneath the vehicle as it was traveling through the Kansas City, Missouri, area. The detonation ldlled the driver and severely injured the driver's wife. (Photo .courtesy of Dan Dyer, the News Leader Newspaper.) Bottom Photo: The scene of an explosion in Duncanville, Alabama, that killed the two occupants of the trailer. 12608.1 U.S. Department of Justice National Institute of Justice This document has been reproduced exactly as received from the person or organization originating it. Points of view or opinions stated In this document are those of the authors and do not necessarily represent the official position or policies of the National Institute of Justice. Permission to reproduce this "'IJIi". material has been granted by U.S. Dept. of Treasury/Bureau of Alcohol, Tobacco & Firearms to the National Criminal Justice Reference Service (NCJRS). Further reproduction outside of the NCJRS system requires permis sion of the ~ owner. Dedication This year we have seen a significant increase in the number of injuries sustained by our State and local counterparts in the law enforcement and fire service communities. The dangers inherent to their line of work are a given; however, the ever-changing environment in which they work has increased these dangers. Booby-trapped drug operations and illicit explosives manufac turing operations are but a few of the criminal activities that present such dangers, not only to them but to the public as well.