Pyrotechnic Serpents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ENCYCLOPEDIA of CHEMISTRY & EXPLOSIVES MATERIALS Abelite
ENCYCLOPEDIA OF CHEMISTRY & EXPLOSIVES MATERIALS A Abelite An explosive, composed mainly of ammonium nitrate and trinitrotoluene. Absolute Zero The least possible temperature for all substances. Generally accepted as -273.15ÝC AC Alternating current. Acceptance Quality Level (AQL) A nominal value expressed in terms of percentage defective per hundred units, by which a group of sampling plans is identified. The sampling plans so identified have a high probability of accepting lots containing material with a process average not greater than the designed value of the AQL. Acetin [CH3COOC3H5(OH)2] also known as glyceryl monoacetate, a colourless hydroscopic liquid. Used as an intermediate for various explosives, and a solvent for various dyes. Acetone [CH3COCH3] colourless, flammable liquid. Acetone is widely used in industry as a solvent for many organic substances. It is used in making synthetic Resins and fillers, smokeless powders, and many other organic compounds. Boiling Point 56ÝC. Useful solvent for acetylene, also known as the simplest saturated ketone. Acetylene or ethyne, a colourless gas and the simplest alkyne Hydrocarbon. Explosive on contact with air, it is stored dissolved under pressure in Acetone. It is used to make neoprene rubber, plastics, and resins. The oxyacetylene torch mixes and burns oxygen and acetylene to produce a very hot flame-as high as 3480ÝC (6300ÝF)-that can cut steel and weld iron and other metals. Produced by the action of wateron calcium carbide and catalytically from naphtha. Acetylide A carbide formed by bubbling acetylene through a metallic salt solution, eg cuprous acetylide, Cu2C2. These are violently explosive compounds. Acid Any substance capable of giving up a proton; a substance that ionizes in solution to give the positive ion of the solvent; a solution with a pH measurement less than 7. -

WPA Newsletter, Volume 25, Issue 1
WPA Newsletter, Volume 25, Issue 1 Table of Contents Corporate Members 4 Editorials 5 Passages 8 Winter Blast Round-Up 12 Burro Races 16 Guest photographer Kelly 18 Maker Faire 27 Cover Picture - “BANG! BANG!” News from Here and There 28 Photo by Kelly Dreller DO IT announcement 31 Elected Officers of the WPA New Rocket Policy 33 President: Steve Wilson Moapa 33 Vice President: Greg Dandurand Blackfinger’s Insert Workshop 34 VP Publications: Pete Hand Treasurer: Richard Haase Secretary: Dennis Miele THE SMALL PRINT The Western Pyrotechnic Association, Inc., also known as the WPA, is a non-profit group of fireworks professionals and their apprentices. This newsletter is a vehicle for their exchange of information in this craft and the right to publish this information is guaranteed by the Constitution of the United States of America. Nonetheless, readers are urged to learn and obey all laws and regulations of all federal, state, and local jurisdictions and of their agencies and representatives. Some information herein may contain incom- plete descriptions of fireworks techniques based on the experience of its author(s) in a controlled environment with circumstances, and conditions different from the reader. Readers must form their own opinion as to the application of this information. This information is considered documentary in nature and no opinion is given as to its suitability or use. No warranties are made either expressed or implied, including but not limited to warranties of the accuracy of the information herein. The WPA is not responsible for the opinions of authors or mistakes in printing. All information is intended solely for viewing by members of the Western Pyrotechnic Association, Inc. -

Black Match” …………………………………………… P
Selected Pyrotechnic Publications of K.L. and B.J. Kosanke Part 5 (1998 through 2000) This book contains 134 pages Development of a Video Spectrometer …………………………………………… P. 435-445. Measurements of Glitter Flash Delay, Size and Duration ……………………… P. 446-449. Lift Charge Loss for a Shell to Remain in Mortar ……………………………… P. 450-450. Configuration and “Over-Load” Studies of Concussion Mortars ……………… P. 451-463. Quick Match – A Review and Study ……………………………………………… P. 464-479. Pyrotechnic Primes and Priming ………………………………………………… P. 480-495. Dud Shell Risk Assessment: NFPA Distances …………………………………… P. 496-499. Dud Shell Risk Assessment: Mortar Placement ………………………………… P. 500-503. Performance Study of Civil War Vintage Black Powder ……………………… P. 504-509. CAUTION: Very Fast “Black Match” …………………………………………… P. 510-512. Peak In-Mortar Aerial Shell Accelerations ……………………………………… P. 513-516. Firing Precision for Choreographed Displays …………………………………… P. 517-518. Sticky Match and Quick Match: Temperature Dependent Burn Times ……… P. 519-523. Mortar Separations in Troughs and Drums …………………………………… P. 524-530. Preliminary Study of the Effect of Ignition Stimulus on Aerial Shell Lift Performance …………………………………………………… P. 531-535. Pyrotechnic Particle Morphologies – Metal Fuels ……………………………… P. 536-542. Peak Mortar Pressures When Firing Spherical Aerial Shells …………………… P. 543-544. Indoor Pyrotechnic Electrostatic Discharge Hazard …………………………… P. 545-545. Pyrotechnic Particle Morphology – Low Melting Point Oxidizers ……………… P. 546-556. An earlier version -
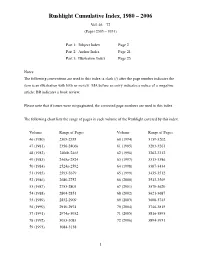
Rushlight Index 1980-2006
Rushlight Cumulative Index, 1980 – 2006 Vol. 46 – 72 (Pages 2305 – 3951) Part 1: Subject Index Page 2 Part 2: Author Index Page 21 Part 3: Illustration Index Page 25 Notes: The following conventions are used in this index: a slash (/) after the page number indicates the item is an illustration with little or no text. MA before an entry indicates a notice of a magazine article; BR indicates a book review. Please note that if issues were mispaginated, the corrected page numbers are used in this index. The following chart lists the range of pages in each volume of the Rushlight covered by this index. Volume Range of Pages Volume Range of Pages 46 (1980) 2305-2355 60 (1994) 3139-3202 47 (1981) 2356-2406a 61 (1995) 3203-3261 48 (1982) 2406b-2465 62 (1996) 3262-3312 49 (1983) 2465a-2524 63 (1997) 3313-3386 50 (1984) 2524a-2592 64 (1998) 3387-3434 51 (1985) 2593-2679 65 (1999) 3435-3512 52 (1986) 2680-2752 66 (2000) 3513-3569 53 (1987) 2753-2803 67 (2001) 3570-3620 54 (1988) 2804-2851 68 (2002) 3621-3687 55 (1989) 2852-2909 69 (2003) 3688-3745 56 (1990) 2910-2974 70 (2004) 3746-3815 57 (1991) 2974a-3032 71 (2005) 3816-3893 58 (1992) 3033-3083 72 (2006) 3894-3951 59 (1993) 3084-3138 1 Rushlight Subject Index Subject Page Andrews' burning fluid vapor lamps 3400-05 Abraham Gesner: Father of Kerosene 2543-47 Andrews patent vapor burner 3359/ Accessories for decorating lamps 2924 Andrews safety lamp, award refused 3774 Acetylene bicycle lamps, sandwich style 3071-79 Andrews, Solomon, 1831 gas generator 3401 Acetylene bicycle lamps, Solar 2993-3004 -

Safety Guidelines of the PGI Official Fireworks Safety Guidelines in Effect at PGI Conventions Pyrotechnics Guild International, Inc
Safety Guidelines of the PGI Official Fireworks Safety Guidelines in effect at PGI Conventions Pyrotechnics Guild International, Inc. 2019 TABLE OF CONTENTS 1. Authority and Scope . 2 2. Safety Committee . 2 3. General Requirements . 3 4. Class B/1.3G Testing . 6 5. Competition . 7 6. Public Display Area . 8 7. Class C/1.4G Sales . 19 8. Class C/1.4G Shooting . 21 9. Auction . 23 10. Assembly of Pyrotechnic Devices . 23 11. Educational Workshops and Demonstrations . 24 12. Trade Show Sales . 24-25 13. Storage . 25 14. Minimum Distance From Spectators . 26 15. Anvil Firing . 28 16. Manufacturing . 28 2019 Committee for Safety Guideline Revision John Steinberg, Chairman Paul Smith Carol Hostetter Jim Beardmore Ruth Newhouse Beardmore 1. AUTHORITY AND SCOPE 1. These guidelines shall be known as the Official Fireworks Safety Guidelines and be cited as such. They shall be referred to herein as “The Guidelines.” 2. Authority: These guidelines were adopted by the Pyrotechnics Guild International, Inc. (PGI) in July 1983; revised July 1987, revised July 1990 [to incorporate changes to NFPA 1123 (1990)], revised June 1994 [to cover the rapid growth of PGI membership]; revised in April 2000 ; revised in May 2001; revised in November of 2008; and revised again in the spring of 2019 and shall remain an official document of the PGI until amended or discontinued. As NFPA codes are continually revised, NFPA-1123, NFPA-1126, and NFPA-160 in their current editions should serve to guide future revisions of these Safety Guidelines. 3. Scope: These Guidelines apply to the handling, storage, sale, discharge or other use of all kinds of fireworks and pyrotechnic or explosive devices during any official PGI convention. -

Washington State Patrol Crime Laboratory Division Materials
Washington State Patrol Crime Laboratory Division Materials Analysis EXPLOSIVES TRAINING MANUAL May 2018 Washington State Patrol Crime Laboratory Division Explosives Laboratory Training Manual Contents 1 INTRODUCTION ..................................................................................................................................... 7 1.1 PURPOSE AND SCOPE ................................................................................................................... 7 1.2 ORGANIZATION OF THE TRAINING MANUAL ................................................................................ 7 2 EXPLOSIVES ANALYSIS OVERVIEW ........................................................................................................ 9 2.1 OBJECTIVES ................................................................................................................................... 9 2.2 OVERVIEW ..................................................................................................................................... 9 2.2.1 Definitions ............................................................................................................................. 9 2.3 SUGGESTED READINGS ............................................................................................................... 11 2.3.1 Introduction ........................................................................................................................ 11 2.3.2 Scene .................................................................................................................................. -

DEFINITIONS and TYPES of CONSUMER FIREWORKS Summary of Survey Responses C.O.B., October 19, 2007
FLORIDA DEPARTMENT OF AGRICULTURE AND CONSUMER SERVICES FLORIDA CONSUMER FIREWORKS TASK FORCE DEFINITIONS AND TYPES OF CONSUMER FIREWORKS Summary of Survey Responses C.O.B., October 19, 2007 Members Responding: Michelle Berger, Rickey Farrell, Tommy Glasgow, Les Hallman, Mike Long, Ira Schwartz Ken Welch Members Not Responding: Trey McCarley, Definition for “Consumer Fireworks” Respondents provided a definition that each would like the Task Force to evaluate at the October 25, 2007 meeting. o Consumer fireworks are those approved on a list maintained by the Florida State Fire Marshals office and which are sold for individual use. o Any small firework device designed to produce visible effects by combustion and which must comply with the construction, chemical composition, and cautionary labeling regulations of the CPSC, as set forth in title 16, Code of Federal Regulations, parts 1500 and 1507. Some small devices designed to produce audible effects are included, such as whistling devices, ground devices containing 50mg or less of explosive materials, and aerial devices containing 130mg or less of explosive materials. The U.S. Dept of Transportation (DOT) at 49 CFR 172.01 classifies consumer fireworks as fireworks UN0336 and UN0337. (Not all fireworks covered by this definition are allowed for consumer use in the State, however may be allowable for wholesale under certain conditions defined within this law.) o Retail purchase of product that does not explode or launch. o The term "consumer fireworks" shall mean and include: Any combustible -

APA STANDARD 87-1 Contents 1
APA STANDARD 87-1 Contents 1. INTRODUCTION..............................................................................................1 2. DEFINITIONS.....................................................................................................1 3. REQUIREMENTS FOR CONSUMER FIREWORKS, NOVELTIES AND THEATRICAL PYROTECHNICS .....................................................................4 3.1 Types of Consumer Fireworks.......................................................................5 3.2 Types of Novelties .........................................................................................8 3.4 Other Devices ................................................................................................9 3.6 Specific Requirements for Consumer Fireworks...........................................10 3.7 Prohibited Chemicals and Components.........................................................12 3.8 Requirements for Theatrical Pyrotechnics ....................................................13 3.9 Approval ........................................................................................................13 3.10 Marking and Labeling..................................................................................14 4. REQUIREMENTS FOR DISPLAY FIREWORKS DEVICES ..........................14 4.1 Types of Display Fireworks Devices.............................................................14 4.2 Construction of Aerial Shells.........................................................................15 4.3 Approval -

Fireworks - Wikipedia, the Free Encyclopedia Page 1 of 17
Fireworks - Wikipedia, the free encyclopedia Page 1 of 17 Fireworks Learn more about using Wikipedia for research. From Wikipedia, the free encyclopedia A firework is classified as a low explosive pyrotechnic device used primarily for aesthetic and entertainment purposes. The most common use of a firework is as part of a fireworks display. A fireworks event (also called a fireworks show or pyrotechnics) is a display of the effects produced by firework devices on various occasions. Fireworks competitions are also regularly held at a number of places. The biggest fireworks event in the world is held in Madeira, Portugal at the New Years' Eve celebrations, as referred in the Guinness World Records. Fireworks (devices) take many forms to produce the four primary effects: noise, light, smoke, and floating materials (confetti for example). They may be designed to burn with colored flames and sparks. Displays are common throughout the world and Fireworks over Miami, Florida, USA on are the focal point of many different cultural and religious celebrations. American Independence Day Fireworks were originally invented by the Chinese, for entertainment purposes, as a natural extension of the Chinese invention of gunpowder. In China, they were first made by firework masters who were well respected for their knowledge of the many complex techniques used to create truly dazzling firework displays. Such important events and festivities as New Year's and the Mid-Autumn Moon Festival were and still are times when fireworks are guaranteed sights. China is the largest manufacturer and exporter of fireworks in the world. China is estimated to have exported over 6 million cases or 120,000 tons of fireworks to the US in 2005.* Fireworks are generally classified as to where they perform, either as a ground or aerial firework. -

Display Fireworks Manual
Display Fireworks Manual 2010 Second Edition 2010 © Her Majesty the Queen in Right of Canada, 2010 Cat. No. M39-127/2010E (Print) ISBN 978-1-100-15116-8 Cat. No. M39-127/2010E-PDF (On-line) ISBN 978-1-100-15117-5 Aussi disponible en français sous le titre : Manuel de l’artificier Table of contents About this manual . v Audience . v Where the manual applies . .vi Where the manual does not apply . vi Authority under the Explosives Act and the Explosives Regulations. vii Amendments and updates . vii Chapter 1 Training and certification . 1 1.1 Display Assistant: duties and certification requirements. 1 1.2 Display Supervisor: duties, restrictions and certification requirements . 2 1.3 Display Supervisor with Endorsements: duties, endorsements and certification requirements. 3 1.4 International Display Supervisors: certification requirements. 4 1.5 Authorities Having Jurisdiction training. 4 Chapter 2 Fireworks and equipment . 5 2.1 Projection- versus emission-type articles . 5 2.2 High-level fireworks. 5 2.3 Low-level fireworks. 9 2.4 Ground-level fireworks. .10 2.5 Chain-fusing methods. 12 Chapter 3 Display site requirements . .15 3.1 Basic requirements. .15 3.2 Minimum distances from the ramp to structures and vehicles . 18 3.3 Minimum distances to overhead objects . .18 3.4 Firing from a flatbed . .19 3.5 Firing from a floating platform. 19 3.6 Obtaining event approval . 22 3.7 Basic requirements for event approval . 22 3.8 Site plan, event description and special circumstances. .22 3.9 Purchasing display fireworks . .23 3.10 Display fireworks event approval form. -

Special Provisions Route 50, Tuckahoe River Bridge
SPECIAL PROVISIONS ROUTE 50, TUCKAHOE RIVER BRIDGE (2E 3B) FEDERAL PROJECT NO. STP-BR-0005(116) AUTHORIZATION OF CONTRACT The Contract is authorized by the provisions of Title 27 of the Revised Statutes of New Jersey and supplements thereto, and Title 23 of the United States Code - Highways. SPECIFICATIONS TO BE USED The 2007 Standard Specifications for Road and Bridge Construction, of the New Jersey Department of Transportation as amended herein will govern the construction of this Project and the execution of the Contract. These Special Provisions consist of the following: Pages 1 to 12 inclusive. General wage determinations issued under Davis-Bacon and related acts, published by US Department of Labor, may be obtained from the Web Determinations online web site at http://www.wdol.gov/dba.aspx#0 Select state, county and construction type heading: HIGHWAY where the Project is to be performed then click Search. Pay the prevailing wage rates determined by the United States Secretary of Labor and the New Jersey Department of Labor. If the prevailing wage rate prescribed for any craft by the United States Secretary of Labor is not the same as the prevailing wage rate prescribed for that craft by the New Jersey Department of Labor, pay the higher rate. State wage rates may be obtained from the New Jersey Department of Labor & Workforce Development (Telephone: 609-292-2259) or by accessing the Department of Labor & Workforce Development’s web site at http://lwd.dol.state.nj.us/labor/wagehour/wagehour_index.html . The State wage rates in effect at the time of award are part of this Contract, pursuant to Chapter 150, Laws of 1963 (NJSA 34:11-56.25, et seq.). -

Wholesale Professional Display CATALOG
Come to our Open House & Product Demo May 18th SEE ALL THE ‘NEW’ LEGAL CONSUMER FIREWORKS FOR MINNESOTA! 2019 PRECOCIOUS PYROTECHNICS Wholesale Professional Display CATALOG PHONE (320)346-2201 or 800-637-4420 FAX (320)346-2403 www.pyro-pro.com [email protected] The Upper Midwest’s Look inside for our NEW complete Largest manufacturer of LANCE & FUSING SYSTEM. Made for Display Fireworks! YOU to save Time and Money! Direct importer of BULK RATE thousands of items from Precocious Pyrotechnics, Inc. (PPI) U. S. POSTAGE all over the world! PAID 4420 - 278th Ave. NW The BEST selection ever! Belgrade, MN 56312-9616 USA Belgrade, MN 56312 Complete line of Supplies, Components, and Finished Fireworks! TO: See us at the 2019 PGII, NFA, WPA and APA conventions. We have it ALL in Both Consumer & Display Fireworks! Precocious Pyrotechnics, Inc. ™ (PPI) 4420 - 278th Avenue NW, Belgrade, MN 56312-9616 USA (320)346-2201 FAX (320)346-2403 STICKLESS ROCKET & BRILLIANT MAGNESIUM METEOR SHELLS (1.3G) TABLE OF CONTENTS Wonderful rising comets! Colors: Silver Glitter or Gold Tremalon/Glitter. CONSUMER FIREWORKS 21-24 Meteors in Colors of Pink, Cherry, Red, Orange, Tangerine, Lemon, DOMESTIC AERIAL DISPLAY FIREWORKS 2-4 Yellow, Gold, Lime, Green, Aqua, Blue, Violet, Purple. Effects can be DOMESTIC GROUND DISPLAY FIREWORKS & LANCE 4-5 mixed and add tailing effects such as Silver or Gold Glitter. FRONTAGE MODULAR FIREWORKS 4 & 18 1" Comet ..................................................................................... $ 6.00 IMPORTED AERIAL DISPLAY TUBE FIREWORKS 9-18 1 ½” Comet ................................................................................. $ 6.60 INFORMATION & DESCRIPTIONS 20 2” Comet ..................................................................................... $ 7.50 MACHINERY 8 2 1/2” Comet ............................................................................... $ 8.50 MATCH, FUSE, PIPE & ELECTRIC MATCH 5-6 3” Comet ....................................................................................