Short Course 11 Pigmented Lesions of the Skin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Features of Benign Tumors of the External Auditory Canal According to Pathology
Central Annals of Otolaryngology and Rhinology Research Article *Corresponding author Jae-Jun Song, Department of Otorhinolaryngology – Head and Neck Surgery, Korea University College of Clinical Features of Benign Medicine, 148 Gurodong-ro, Guro-gu, Seoul, 152-703, South Korea, Tel: 82-2-2626-3191; Fax: 82-2-868-0475; Tumors of the External Auditory Email: Submitted: 31 March 2017 Accepted: 20 April 2017 Canal According to Pathology Published: 21 April 2017 ISSN: 2379-948X Jeong-Rok Kim, HwibinIm, Sung Won Chae, and Jae-Jun Song* Copyright Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College © 2017 Song et al. of Medicine, South Korea OPEN ACCESS Abstract Keywords Background and Objectives: Benign tumors of the external auditory canal (EAC) • External auditory canal are rare among head and neck tumors. The aim of this study was to analyze the clinical • Benign tumor features of patients who underwent surgery for an EAC mass confirmed as a benign • Surgical excision lesion. • Recurrence • Infection Methods: This retrospective study involved 53 patients with external auditory tumors who received surgical treatment at Korea University, Guro Hospital. Medical records and evaluations over a 10-year period were examined for clinical characteristics and pathologic diagnoses. Results: The most common pathologic diagnoses were nevus (40%), osteoma (13%), and cholesteatoma (13%). Among the five pathologic subgroups based on the origin organ of the tumor, the most prevalent pathologic subgroup was the skin lesion (47%), followed by the epithelial lesion (26%), and the bony lesion (13%). No significant differences were found in recurrence rate, recurrence duration, sex, or affected side between pathologic diagnoses. -

Acral Compound Nevus SJ Yun S Korea
University of Pennsylvania, Founded by Ben Franklin in 1740 Disclosures Consultant for Myriad Genetics and for SciBase (might try to sell you a book, as well) Multidimensional Pathway Classification of Melanocytic Tumors WHO 4th Edition, 2018 Epidemiologic, Clinical, Histologic and Genomic Aspects of Melanoma David E. Elder, MB ChB, FRCPA University of Pennsylvania, Philadelphia, PA, USA Napa, May, 2018 3rd Edition, 2006 Malignant Melanoma • A malignant tumor of melanocytes • Not all melanomas are the same – variation in: – Epidemiology – risk factors, populations – Cell/Site of origin – Precursors – Clinical morphology – Microscopic morphology – Simulants – Genomic abnormalities Incidence of Melanoma D.M. Parkin et al. CSD/Site-Related Classification • Bastian’s CSD/Site-Related Classification (Taxonomy) of Melanoma – “The guiding principles for distinguishing taxa are genetic alterations that arise early during progression; clinical or histologic features of the primary tumor; characteristics of the host, such as age of onset, ethnicity, and skin type; and the role of environmental factors such as UV radiation.” Bastian 2015 Epithelium associated Site High UV Low UV Glabrous Mucosa Benign Acquired Spitz nevus nevus Atypical Dysplastic Spitz Borderline nevus tumor High Desmopl. Low-CSD Spitzoid Acral Mucosal Malignant CSD melanoma melanoma melanoma melanoma melanoma 105 Point mutations 103 Structural Rearrangements 2018 WHO Classification of Melanoma • Integrates Epidemiologic, Genomic, Clinical and Histopathologic Features • Assists -
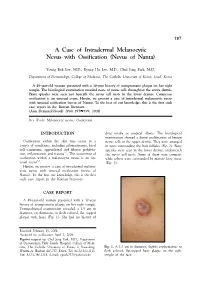
A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta)
197 A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta) Young Bok Lee, M.D., Kyung Ho Lee, M.D., Chul Jong Park, M.D. Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea A 49-year-old woman presented with a 30-year history of asymptomatic plaque on her right temple. The histological examination revealed nests of nevus cells throughout the entire dermis. Bony spicules were seen just beneath the nevus cell nests in the lower dermis. Cutaneous ossification is an unusual event. Herein, we present a case of intradermal melanocytic nevus with unusual ossification (nevus of Nanta). To the best of our knowledge, this is the first such case report in the Korean literature. (Ann Dermatol (Seoul) 20(4) 197∼199, 2008) Key Words: Melanocytic nevus, Ossification INTRODUCTION drug intake or medical illness. The histological examination showed a dense proliferation of benign Ossification within the skin may occur in a nevus cells in the upper dermis. They were arranged variety of conditions, including pilomatricoma, basal in nests surrounding the hair follicles (Fig. 2). Bony cell carcinoma, appendageal and fibrous prolifera- spicules were seen in the lower dermis, underneath 1,2 tion, inflammation and trauma . The occurrence of the nevus cell nests. Some of them were compact ossification within a melanocytic nevus is an un- while others were surrounded by mature fatty tissue 3-5 usual event . (Fig. 3). Herein, we present a case of intradermal melano- cytic nevus with unusual ossification (nevus of Nanta). To the best our knowledge, this is the first such case report in the Korean literature. -

Oral Pathology
Oral Pathology Palatal blue nevus in a child Catherine M. Flaitz DDS, MS Georgeanne McCandless DDS Dr. Flaitz is professor, Oral and Maxillofacial Pathology and Pediatric Dentistry, Department of Stomatology, University of Texas at Houston Health Science Center Dental Branch; Dr. McCandless has a private practice in The Woodlands, TX. Correspond with Dr. Flaitz at [email protected] Abstract The intraoral blue nevus occurs infrequently in children. This by the labial mucosa (1). Intraoral lesions have a predilection case report describes the clinical features of an acquired blue ne- for females in the third and fourth decades, in contrast to cu- vus in a 7 year-old girl that involved the palatal mucosa. A taneous lesions that normally develop in children. In large differential diagnosis and justification for surgical excision of this biopsy series, only 2% of the oral blue nevi are diagnosed in oral lesion are discussed. (Pediatr Dent 23:354-355, 2001) children and adolescents (1). Similar to their cutaneous coun- terpart, most oral lesions are acquired; however, there are ith the exception of vascular entities, neoplastic isolated reports of congenital examples. lesions with a blue discoloration are an unusual find Clinically, most lesions present as a solitary blue, gray or Wing in children. Although the blue nevus is a blue-black macule or slightly raised nodule that measures less relatively common finding of the skin in the pediatric popula- than 6 mm in size. The margins are often regular but indis- tion, only a few intraoral examples are documented in the tinct and the surface is smooth. -

Identification of HRAS Mutations and Absence of GNAQ Or GNA11
Modern Pathology (2013) 26, 1320–1328 1320 & 2013 USCAP, Inc All rights reserved 0893-3952/13 $32.00 Identification of HRAS mutations and absence of GNAQ or GNA11 mutations in deep penetrating nevi Ryan P Bender1, Matthew J McGinniss2, Paula Esmay1, Elsa F Velazquez3,4 and Julie DR Reimann3,4 1Caris Life Sciences, Phoenix, AZ, USA; 2Genoptix Medical Laboratory, Carlsbad, CA, USA; 3Dermatopathology Division, Miraca Life Sciences Research Institute, Newton, MA, USA and 4Department of Dermatology, Tufts Medical Center, Boston, MA, USA HRAS is mutated in B15% of Spitz nevi, and GNAQ or GNA11 is mutated in blue nevi (46–83% and B7% respectively). Epithelioid blue nevi and deep penetrating nevi show features of both blue nevi (intradermal location, pigmentation) and Spitz nevi (epithelioid morphology). Epithelioid blue nevi and deep penetrating nevi can also show overlapping features with melanoma, posing a diagnostic challenge. Although epithelioid blue nevi are considered blue nevic variants, no GNAQ or GNA11 mutations have been reported. Classification of deep penetrating nevi as blue nevic variants has also been proposed, however, no GNAQ or GNA11 mutations have been reported and none have been tested for HRAS mutations. To better characterize these tumors, we performed mutational analysis for GNAQ, GNA11, and HRAS, with blue nevi and Spitz nevi as controls. Within deep penetrating nevi, none demonstrated GNAQ or GNA11 mutations (0/38). However, 6% revealed HRAS mutation (2/32). Twenty percent of epithelioid blue nevi contained a GNAQ mutation (2/10), while none displayed GNA11 or HRAS mutation. Eighty-seven percent of blue nevi contained a GNAQ mutation (26/30), 4% a GNA11 mutation (1/28), and none an HRAS mutation. -

Lumps & Bumps: Approach to Common Dermatologic Neoplasms
Case-Based Approach to Common Dermatologic Neoplasms Patrick Retterbush, MD, FAAD Mohs Surgery & Dermatologic Oncology Associate Member of the American College of Mohs Surgery Private Practice: Lockman Dermatology January 27th 2018 Disclosure of Relevant Financial Relationships • I do not have any relevant financial relationships, commercial interests, and/or conflicts of interest regarding the content of this presentation. Goals/Objectives • Recognize common benign growths • Recognize common malignant growths • Useful clues & examination for evaluating melanocytic nevi and when to be concerned for melanoma/atypical moles • How to perform a basic skin biopsy and which method/type to choose • Basic treatment/when to refer Key Questions & Physical Examination Findings for a Growth History Physical Examination • How long has the lesion been • Describing a growth present? – flat or raised? • flat – macule (<1cm) or patch (>1cm) – years, months, weeks • raised – papule (<1cm) or plaque (>1cm) – nodule if deep (majority of lesion in • Has it changed? dermis/SQ) – Size – secondary descriptive features • scaly (hyperkeratosis, retention of strateum – Shape corneum) – Color • crusty (dried serum, blood, or pus on surface) • eroded or ulcerated (partial vs. full thickness – Symptoms – pain, bleeding, itch? epidermal loss) – Over what time frame? • color (skin colored, red, pigmented, pearly) • feel (hard or soft, mobile or fixed) • PMH: • size: i.e. 6 x 4mm – prior skin cancers • Look at the rest of the skin/region of skin • SCC/BCCs vs. melanoma -

Acral Melanoma
Accepted Date : 07-Jul-2015 Article type : Original Article The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma Running head: Dermoscopy of acral melanoma Word count: 3138, Tables: 6, Figures: 6 A. Lallas,1 A. Kyrgidis,1 H. Koga,2 E. Moscarella,1 P. Tschandl,3 Z. Apalla,4 A. Di Stefani,5 D. Ioannides,2 H. Kittler,4 K. Kobayashi,6,7 E. Lazaridou,2 C. Longo,1 A. Phan,8 T. Saida,3 M. Tanaka,6 L. Thomas,8 I. Zalaudek,9 G. Argenziano.10 Article 1. Skin Cancer Unit, Arcispedale Santa Maria Nuova IRCCS, Reggio Emilia, Italy 2. Department of Dermatology, Shinshu University School of Medicine, Matsumoto, Japan 3. Department of Dermatology, Division of General Dermatology, Medical University, Vienna, Austria 4. First Department of Dermatology, Medical School, Aristotle University, Thessaloniki, Greece 5. Division of Dermatology, Complesso Integrato Columbus, Rome, Italy 6. Department of Dermatology, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan 7. Kobayashi Clinic, Tokyo, Japan 8. Department of Dermatology, Claude Bernard - Lyon 1 University, Centre Hospitalier Lyon-Sud, Pierre Bénite, France. 9. Department of Dermatology and Venereology, Medical University, Graz, Austria 10. Dermatology Unit, Second University of Naples, Naples, Italy. This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as Accepted doi: 10.1111/bjd.14045 This article is protected by copyright. All rights reserved. Please address all correspondence to: Aimilios Lallas, MD. -

The State of the Dysplastic Nevus in the 21St Century
The State of the Dysplastic Nevus in the 21st Century Keith Duffy, MD Associate professor Department of Dermatology University of Utah Department of Dermatology Disclosures • Myriad Genetics – Advisory board; honorarium • Castle Biosciences – Advisory board; honorarium What do I do? • Clinical – 60% Mohs micrographic and reconstructive surgery and high risk skin cancer – 40% Dermatopathology sign-out – Multidisciplinary cutaneous oncology program – Huntsman Cancer Institute • Administrative – Residency Program Director, Dermatology Department of Dermatology Department of Dermatology The current(ish) state of affairs… Do you believe dysplastic (Clark) nevi are truly premalignant lesions? 53% A. Yes B. No 25% C. Unsure 22% A. B. C. How do you report “dysplastic nevi”? A. Dysplastic nevus 62% B. Clark nevus C. Nevus with architectural disorder 19% D. Other 12% 7% A. B. C. D. Do you assign a histologic “grade” to these nevi? 87% A. Yes B. No 13% A. B. If yes, what grading system do you use? A. Cytology as three grades (mild, moderate, 73% severe) B. Cytology and architecture as two separate grades C. Cytology as two grades 8% 10% 10% only D. Other grading system A. B. C. D. Brief history • 1978 – Dr. Clark describes nevi associated with melanoma prone families – The B-K mole syndrome • 1978 – Dr. Lynch describes a single multi- generational family with melanoma and nevi – Familial atypical multiple mole melanoma syndrome (FAMMM) Brief history • 1980 – Dr. Elder and Clark describe ‘dysplastic nevi’ in a non-familial setting – Introduction of the term ‘dysplastic nevus syndrome’ • Familial and sporadic variants • Formally postulated that ‘dysplastic nevi’ are precursors of melanoma Dr. -

Lentigo Maligna Melanoma and Simulants Maui January 2020 Superficial Atypical Melanocytic Proliferations
Superficial Atypical Melanocytic Proliferations II. Lentigo Maligna Melanoma and Simulants Maui January 2020 Superficial Atypical Melanocytic Proliferations • RGP Melanomas • SSM, LMM, ALM, MLM • Intermediate lesions • Dysplastic nevi, Atypical lentiginous proliferations in high CSD skin; Atypical Acral lentiginous nevi • Superficial atypical melanocytic proliferations • Pagetoid plaque-like Spitz nevi; pigmented spindle cell nevus (Reed) • Special site nevi (genital, breast, scalp, ear, flexural, etc). • Superficial atypical melanocytic proliferations of uncertain significance • Atypical/unusual/uncertain examples of all of the above Superficial Atypical Melanocytic Proliferations • RGP Melanomas • SSM, LMM, ALM, MLM • Intermediate lesions • Dysplastic nevi, Atypical lentiginous proliferations in high CSD skin; Atypical Acral lentiginous nevi • Superficial atypical melanocytic proliferations • Pagetoid plaque-like Spitz nevi; pigmented spindle cell nevus (Reed) • Special site nevi (genital, breast, scalp, ear, flexural, etc). • Superficial atypical melanocytic proliferations of uncertain significance • Atypical/unusual/uncertain examples of all of the above High CSD Melanomas and Simulants. D Elder, Maui, HI Jan 2020 Lentigo maligna melanoma Atypical lentiginous nevi/proliferations High CSD: Lentiginous Nevi and Lentigo Maligna Melanoma and Simulant(s) • Lentiginous Melanoma of Sun-Damaged Skin • LMM in situ • LMM invasive • Distinction from Dysplastic Nevi (Dysplastic Nevus-like Melanoma/Nevoid Lentigo Maligna • Lentiginous Nevi of -

Dysplastic Nevus Panel Discussion
Dysplastic Nevus Panel Discussion Maxwell Fung, MD, Moderator Director, UC Davis Dermatopathology Service Kerri Rieger, MD PhD Stanford Dermatopathology Service Joshua Schulman, MD Director of Dermatopathology, Sacramento Veterans Affairs Medical Center Definitions, Diagnostic criteria Dysplastic nevus • Major criteria (required) • basilar proliferation of atypical melanocytes extending 3 rete ridges beyond a dermal component (if present) i.e. “shoulder” • intraepidermal melanocytic proliferation (lentiginous or epithelioid) • Minor criteria (≥ 2) • fusion of rete ridges • concentric/lamellar eosinophilic fibrosis • inflammatory host response • neovascularization Clemente C, et al. Histopathologic diagnosis of dysplastic nevi: concordance among pathologists convened by the WHO Melanoma Programme. Hum Pathol 1991;22:313-19. Naeyaert JM, Brochez L. Dysplastic nevi. N Eng J Med 2003;23:349:2233-2240 Atypical nevus/mole Fig 1. Duffy K, Grossman D. • Usually 4-12 mm The dysplastic nevus. J Am Acad Dermatol 2012;67:19:31-12. • Asymmetry • Irregular pigmentation • Irregular border pigmented • Ill-defined border seborrheic keratosis • Macular component, usually peripheral Dysplastic nevus syndrome atypical mole syndrome, B-K mole syndrome, familial melanoma syndrome, familial atypical multiple mole-melanoma (FAMMM) • OMIM #155600 NIH Consensus criteria Occurrence of melanoma in ≥1 first- or second-degree relatives Large number of nevi (often >50), some of which are clinically atypical (and) Nevi with certain distinct histologic features Dutch -

Nevus of Ota in Children
PEDIATRIC DERMATOLOGY Series Editor: Camila K. Janniger, MD Nevus of Ota in Children Smeeta Sinha, MD; Philip J. Cohen, MD; Robert A. Schwartz, MD, MPH Nevus of Ota, synonymously termed oculodermal seen most commonly in individuals of Japanese melanosis, is an uncommon dermal melanosis descent, and is less likely to present in individuals most commonly seen at birth in children of of Chinese or Korean descent, though individuals Japanese descent, though it can affect individu- descending from the Indian subcontinent, Africa, als of any age or ethnicity. The disease tends to and Europe also may be affected.7 In early sur- persist and extend locally, becoming increasingly veys of Japanese patients at dermatology clinics, prominent with age, puberty, and postmenopausal the incidence of nevus of Ota was determined to state. Treatment should begin early after diagno- be 0.4% (110/27,500).4 Cowan and Balistocky8 sis using multiple sessions of laser photother- calculated the incidence of oculodermal melano- molysis to avoid darkening and extension of the cytosis in black patients to be 0.016%. A study of lesion. Important associated disorders include 2914 Chinese children in Calgary, Alberta, Canada, ipsilateral glaucoma; intracranial melanocyto- reported an incidence of oculodermal melanocytosis sis; and rarely cutaneous, ocular, or intracranial of 0.034% (1/2914).9 melanoma. Recommendations are discussed for managing nevus of Ota in children. Clinical Manifestation Cutis. 2008;82:25-29. The typical nevus of Ota is a unilateral facial dis- coloration that is macular, speckled, and bluish gray or brown, with edges that blend with bordering skin evus of Ota is a rare disorder characterized (Figure).10 The dermatomal distribution of pigment by melanocytic pigmentation of the sclera characterizes this diagnosis in most cases. -

Blue Nevi and Melanomas Natural Blue BLUE NEVUS Blue Nevus (BN)
KJ Busam, M.D. Paris, 2017 Blue Nevi and Melanomas Natural Blue BLUE NEVUS Blue Nevus (BN) • Spectrum of blue nevi – Common, Sclerosing, Epithelioid, Cellular, Plaque type blue nevi • Differential diagnosis – Melanoma ex BN or simulating BN – BN vs other tumors – Biphenotypic/collision lesions Common Blue Nevus Clinical: - Circumscribed small bluish macule/papule - Preferred sites: Scalp, wrist, foot Pathology: - Predominantly reticular dermal lesion - Pigmented fusiform and dendritic cells - Admixed melanophages - Bland cytology Common Blue Nevus Blue Nevus Sclerosing Blue Nevus Pigm BN Cellular Blue Nevus - 49 yo woman - Buttock nodule CBN Cellular Blue Nevus Thrombi and stromal edema Multinucleated giant melanocytes Cellular Blue Nevus Hemorrhagic cystic (“aneurysmal”) change Amelanotic Cellular Blue Nevus 19 yo man with buttock lesion Atypical CBN Plaque-Type Blue Nevus Plaque-type Blue Nevus Plaque Type Blue Nevus Mucosal Blue Nevus Conjunctival Blue Nevus Nodal Blue Nevus Combined epithelioid BN Blue Nevus • M Tieche 1906; Virchow Arch Pathol Anat “Blaue Naevus” • B Upshaw 1947; Surgery “Extensive Blue Nevus” (plaque-type BN) • A Allen 1949; Cancer “ Cellular Blue Nevus” Blue Nevus – Mutation Analysis Type of Lesion GNAQ GNA11 Number Common BN 6.7% 65% 60 Cellular BN 8.3% 72.2% 36 Amelanotic BN 0% 70% 10 Nevus of Ota 5% 10% 20 Nevus of Ito 16.7% 0% 7 TOTAL 6.5% 55% 139 Van Raamsdonk et al NEJM 2010; 2191-9 Blue Nevus – Mutation Analysis Type of Blue Nevus GNAQ Number Common Blue Nevus 40% 4/10 Cellular Blue Nevus 44% 4/9 Hypomelanotic