Acral Compound Nevus SJ Yun S Korea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Features of Benign Tumors of the External Auditory Canal According to Pathology
Central Annals of Otolaryngology and Rhinology Research Article *Corresponding author Jae-Jun Song, Department of Otorhinolaryngology – Head and Neck Surgery, Korea University College of Clinical Features of Benign Medicine, 148 Gurodong-ro, Guro-gu, Seoul, 152-703, South Korea, Tel: 82-2-2626-3191; Fax: 82-2-868-0475; Tumors of the External Auditory Email: Submitted: 31 March 2017 Accepted: 20 April 2017 Canal According to Pathology Published: 21 April 2017 ISSN: 2379-948X Jeong-Rok Kim, HwibinIm, Sung Won Chae, and Jae-Jun Song* Copyright Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College © 2017 Song et al. of Medicine, South Korea OPEN ACCESS Abstract Keywords Background and Objectives: Benign tumors of the external auditory canal (EAC) • External auditory canal are rare among head and neck tumors. The aim of this study was to analyze the clinical • Benign tumor features of patients who underwent surgery for an EAC mass confirmed as a benign • Surgical excision lesion. • Recurrence • Infection Methods: This retrospective study involved 53 patients with external auditory tumors who received surgical treatment at Korea University, Guro Hospital. Medical records and evaluations over a 10-year period were examined for clinical characteristics and pathologic diagnoses. Results: The most common pathologic diagnoses were nevus (40%), osteoma (13%), and cholesteatoma (13%). Among the five pathologic subgroups based on the origin organ of the tumor, the most prevalent pathologic subgroup was the skin lesion (47%), followed by the epithelial lesion (26%), and the bony lesion (13%). No significant differences were found in recurrence rate, recurrence duration, sex, or affected side between pathologic diagnoses. -

CASE REPORT Intradermal Nevus of the External Auditory Canal
Int. Adv. Otol. 2009; 5:(3) 401-403 CASE REPORT Intradermal Nevus of the External Auditory Canal: A Case Report Sedat Ozturkcan, Ali Ekber, Riza Dundar, Filiz Gulustan, Demet Etit, Huseyin Katilmis Department of Otorhinolaryngology and Head and Neck Surgery ‹zmir Atatürk Research and Training Hospital, Ministry of Health, ‹ZM‹R-TURKEY (SO, AE, FG, DE, HK) Department of Otorhinolaryngology and Head and Neck Surgery Etimesgut Military Hospital , ANKARA-TURKEY (RD) Intradermal nevus is the most common skin tumor in humans; however, its occurrence in the external auditory canal (EAC) is uncommon. The clinical manifestations of pigmented nevus of the EAC have been reported to include ear fullness, foreign body sensation, hearing impairment, and otalgia, but some cases were asymptomatic and were found incidentally. The treatment of choice for a symptomatic intradermal nevus in the EAC is complete excision. There has been no recurrence reported in the literature . A pedunculated, papillomatous hair-bearing lesion was detected in the external auditory canal of the patient who was on follow-up for pruritus. Clinical and pathologic features of an intradermal nevus of the external auditory canal are presented, and the literature reviewed. Submitted : 14 October 2008 Revised : 01 July 2009 Accepted : 09 July 2009 Intradermal nevus is the most common skin tumor in left external auditory canal. Otomicroscopic humans; however, its occurrence in the external examination revealed a pedunculated, papillomatous auditory canal (EAC) is uncommon [1-4]. Intradermal hair-bearing lesion in the postero-inferior cartilaginous nevus is considered to be a form of benign cutaneous portion of the external auditory canal (Figure 1). -
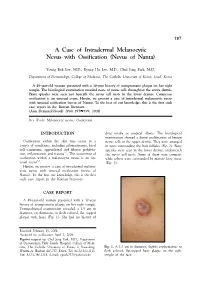
A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta)
197 A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta) Young Bok Lee, M.D., Kyung Ho Lee, M.D., Chul Jong Park, M.D. Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea A 49-year-old woman presented with a 30-year history of asymptomatic plaque on her right temple. The histological examination revealed nests of nevus cells throughout the entire dermis. Bony spicules were seen just beneath the nevus cell nests in the lower dermis. Cutaneous ossification is an unusual event. Herein, we present a case of intradermal melanocytic nevus with unusual ossification (nevus of Nanta). To the best of our knowledge, this is the first such case report in the Korean literature. (Ann Dermatol (Seoul) 20(4) 197∼199, 2008) Key Words: Melanocytic nevus, Ossification INTRODUCTION drug intake or medical illness. The histological examination showed a dense proliferation of benign Ossification within the skin may occur in a nevus cells in the upper dermis. They were arranged variety of conditions, including pilomatricoma, basal in nests surrounding the hair follicles (Fig. 2). Bony cell carcinoma, appendageal and fibrous prolifera- spicules were seen in the lower dermis, underneath 1,2 tion, inflammation and trauma . The occurrence of the nevus cell nests. Some of them were compact ossification within a melanocytic nevus is an un- while others were surrounded by mature fatty tissue 3-5 usual event . (Fig. 3). Herein, we present a case of intradermal melano- cytic nevus with unusual ossification (nevus of Nanta). To the best our knowledge, this is the first such case report in the Korean literature. -

Short Course 11 Pigmented Lesions of the Skin
Rev Esp Patol 1999; Vol. 32, N~ 3: 447-453 © Prous Science, SA. © Sociedad Espafiola de Anatomfa Patol6gica Short Course 11 © Sociedad Espafiola de Citologia Pigmented lesions of the skin Chairperson F Contreras Spain Ca-chairpersons S McNutt USA and P McKee, USA. Problematic melanocytic nevi melanin pigment is often evident. Frequently, however, the lesion is solely intradermal when it may be confused with a fibrohistiocytic RH. McKee and F.R.C. Path tumor, particularly epithelloid cell fibrous histiocytoma (4). It is typi- cally composed of epitheliold nevus cells with abundant eosinophilic Brigham and Women’s Hospital, Harvard Medical School, Boston, cytoplasm and large, round, to oval vesicular nuclei containing pro- USA. minent eosinophilic nucleoli. Intranuclear cytoplasmic pseudoinclu- sions are common and mitotic figures are occasionally present. The nevus cells which are embedded in a dense, sclerotic connective tis- Whether the diagnosis of any particular nevus is problematic or not sue stroma, usually show maturation with depth. Less frequently the nevus is composed solely of spindle cells which may result in confu- depends upon a variety of factors, including the experience and enthusiasm of the pathologist, the nature of the specimen (shave vs. sion with atrophic fibrous histiocytoma. Desmoplastic nevus can be distinguished from epithelloid fibrous histiocytoma by its paucicellu- punch vs. excisional), the quality of the sections (and their staining), larity, absence of even a focal storiform growth pattern and SiQO pro- the hour of the day or day of the week in addition to the problems relating to the ever-increasing range of histological variants that we tein/HMB 45 expression. -

Optimal Management of Common Acquired Melanocytic Nevi (Moles): Current Perspectives
Clinical, Cosmetic and Investigational Dermatology Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Optimal management of common acquired melanocytic nevi (moles): current perspectives Kabir Sardana Abstract: Although common acquired melanocytic nevi are largely benign, they are probably Payal Chakravarty one of the most common indications for cosmetic surgery encountered by dermatologists. With Khushbu Goel recent advances, noninvasive tools can largely determine the potential for malignancy, although they cannot supplant histology. Although surgical shave excision with its myriad modifications Department of Dermatology and STD, Maulana Azad Medical College and has been in vogue for decades, the lack of an adequate histological sample, the largely blind Lok Nayak Hospital, New Delhi, Delhi, nature of the procedure, and the possibility of recurrence are persisting issues. Pigment-specific India lasers were initially used in the Q-switched mode, which was based on the thermal relaxation time of the melanocyte (size 7 µm; 1 µsec), which is not the primary target in melanocytic nevus. The cluster of nevus cells (100 µm) probably lends itself to treatment with a millisecond laser rather than a nanosecond laser. Thus, normal mode pigment-specific lasers and pulsed ablative lasers (CO2/erbium [Er]:yttrium aluminum garnet [YAG]) are more suited to treat acquired melanocytic nevi. The complexities of treating this disorder can be overcome by following a structured approach by using lasers that achieve the appropriate depth to treat the three subtypes of nevi: junctional, compound, and dermal. Thus, junctional nevi respond to Q-switched/normal mode pigment lasers, where for the compound and dermal nevi, pulsed ablative laser (CO2/ Er:YAG) may be needed. -

Case Report of Giant Congenital Melanocytic Nevus
PEDIATRIC DERMATOLOGY Series Editor: Camila K. Janniger, MD Bathing Trunks Nevus: Case Report of Giant Congenital Melanocytic Nevus Ronald Russ, DO; Lisa Light, MS-IV Bathing trunks nevi, a subtype of giant congeni- 34 years. All maternal and prenatal history was unre- tal melanocytic nevi (CMN), are skin tumors that markable. Upon initial physical examination as a new- present by 2 years of age and occur in a low born (1 hour following delivery), the infant had a large percentage of all births. We report a case of (≥5% body surface area), circumferentially pigmented bathing trunks nevus that was initially suspected area from the umbilicus to mid thigh bilaterally to be melanoma, and describe the history, patho- (Figure 1). Interposed darkened lesions were pres- physiology, and treatment options for CMN. We ent, with 3 distinct, raised, lipomatous-type nodules also discuss the risk for neurocutaneous melano- (2 cm, 1.3 cm, and 3 cm in diameter from left to right) sis (NCM), which is a rare syndrome in patients over the lower lumbar spine (Figure 2). There were no with giant CMN. signs of jaundice, hemolysis, meningomyelocele, or Cutis. 2009;83:69-72. abnormal hair growth. The rest of the physical exami- nation was unremarkable, including cardiovascular, pulmonary, and abdominal systems, and genitourinary athing trunks nevus is a specific subtype of functioning was normal. Cord blood testing revealed giant congenital melanocytic nevus (CMN) A Rh-positive blood type, and a direct Coombs test B with spread resembling bathing trunks. This was negative for antibodies. Complete blood cell rare variant is clinically significant because of the count was within reference range, with the excep- increased risk for progression to melanoma and its tion of a low platelet count of 2343103/µL (reference association with neurocutaneous melanosis (NCM).1 range, 250–4503103/µL). -

Acral Melanoma Toshiaki Saida, Hiroshi Koga, Yoriko Yamazaki, Masaru Tanaka IV.2
Chapter IV.2 Acral Melanoma Toshiaki Saida, Hiroshi Koga, Yoriko Yamazaki, Masaru Tanaka IV.2 Contents thickness, biological behavior is not different among the four histogenetic types [16]. More- IV.2.1 Definition . .196 over, cutaneous melanomas not infrequently IV.2 IV.2.2 Clinical Features . .197 show overlapping histopathological features of IV2.3 Dermoscopic Criteria. 198 the four types [36]. Ackerman repeatedly criti- cized the validity of the Clark’s classification IV.2.4 Relevant Clinical Differential and proposed the unifying concept of melano- Diagnosis. 198 ma [1]. IV.2.5 Histopathology. .199 Recently, Bastian and co-workers defined ac- ral melanoma as melanoma occurring on the IV.2.6 Management. .200 non-hair-bearing skin of the palms or soles or IV.2.7 Case Study. .200 under the nails and found that this type of mel- References. .202 anoma was unique in frequent amplifications of chromosomes 5p15, 5p13, 11q13, and 12q14 [4, 7]. Particularly, amplification of 11q13 was de- tected in ~50% of this type of melanoma. Cyclin D1 is the most important candidate gene located in this chromosome region. It is noteworthy IV.2.1 Definition that 5 of 36 acral melanomas defined by Bastian and co-workers were superficial spreading mel- Acral melanoma is a melanoma that affects ac- anoma according to Clark’s classification [7]. ral areas of the skin, which is the most prevalent Another characteristic of acral melanoma is site of melanoma in non-Caucasians [5, 10]. very low rate of mutation of the BRAF onco- Strictly speaking, acral lentiginous melanoma is gene, which is commonly found in superficial not a synonym for acral melanoma. -

Congenital Melanocytic Nevus
BCCH Pediatric Dermatology Clinic Joseph M Lam, MD CONGENITAL MELANOCYTIC NEVUS What is a congenital melanocytic nevus? A "congenital melanocytic nevus" (aka mole) is the name for a common brown birthmark which is made up of special pigment-producing cells. The size of the birthmark may range from a small 1 cm mark to a giant birthmark covering half of the body or more. How common are congenital melanocytic nevus? Small congenital pigmented moles (brown birthmarks) are seen in 1 percent of all healthy newborn babies. Giant congenital moles (larger than 8 inches) are rare, found in fewer than one in 20,000 newborn infants. Why are they special? Small- and medium-sized congenital moles may rarely develop melanoma, a worrisome form of skin cancer. However, the risk of this happening is less than 1% and in adults, the risk of developing skin cancer in any area of the skin is much higher than the risk of melanoma in a small or medium-sized congenital mole. However, depending upon the appearance of the mole, its location and the ease of removal, we may suggest that the mole be taken out or we may recommend keeping the mole and just paying attention to any changes in the mole. Rarely, mole cells can be present in the brain - this happens in patients with many ‘satellite’ moles. If this causes problems, the problems usually show up in the first few months of life. It is important to inspect congenital moles on a regular basis at home. We may also recommend that some moles be observed in the office with pictures. -

Lumps & Bumps: Approach to Common Dermatologic Neoplasms
Case-Based Approach to Common Dermatologic Neoplasms Patrick Retterbush, MD, FAAD Mohs Surgery & Dermatologic Oncology Associate Member of the American College of Mohs Surgery Private Practice: Lockman Dermatology January 27th 2018 Disclosure of Relevant Financial Relationships • I do not have any relevant financial relationships, commercial interests, and/or conflicts of interest regarding the content of this presentation. Goals/Objectives • Recognize common benign growths • Recognize common malignant growths • Useful clues & examination for evaluating melanocytic nevi and when to be concerned for melanoma/atypical moles • How to perform a basic skin biopsy and which method/type to choose • Basic treatment/when to refer Key Questions & Physical Examination Findings for a Growth History Physical Examination • How long has the lesion been • Describing a growth present? – flat or raised? • flat – macule (<1cm) or patch (>1cm) – years, months, weeks • raised – papule (<1cm) or plaque (>1cm) – nodule if deep (majority of lesion in • Has it changed? dermis/SQ) – Size – secondary descriptive features • scaly (hyperkeratosis, retention of strateum – Shape corneum) – Color • crusty (dried serum, blood, or pus on surface) • eroded or ulcerated (partial vs. full thickness – Symptoms – pain, bleeding, itch? epidermal loss) – Over what time frame? • color (skin colored, red, pigmented, pearly) • feel (hard or soft, mobile or fixed) • PMH: • size: i.e. 6 x 4mm – prior skin cancers • Look at the rest of the skin/region of skin • SCC/BCCs vs. melanoma -

Acral Melanoma
Accepted Date : 07-Jul-2015 Article type : Original Article The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma Running head: Dermoscopy of acral melanoma Word count: 3138, Tables: 6, Figures: 6 A. Lallas,1 A. Kyrgidis,1 H. Koga,2 E. Moscarella,1 P. Tschandl,3 Z. Apalla,4 A. Di Stefani,5 D. Ioannides,2 H. Kittler,4 K. Kobayashi,6,7 E. Lazaridou,2 C. Longo,1 A. Phan,8 T. Saida,3 M. Tanaka,6 L. Thomas,8 I. Zalaudek,9 G. Argenziano.10 Article 1. Skin Cancer Unit, Arcispedale Santa Maria Nuova IRCCS, Reggio Emilia, Italy 2. Department of Dermatology, Shinshu University School of Medicine, Matsumoto, Japan 3. Department of Dermatology, Division of General Dermatology, Medical University, Vienna, Austria 4. First Department of Dermatology, Medical School, Aristotle University, Thessaloniki, Greece 5. Division of Dermatology, Complesso Integrato Columbus, Rome, Italy 6. Department of Dermatology, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan 7. Kobayashi Clinic, Tokyo, Japan 8. Department of Dermatology, Claude Bernard - Lyon 1 University, Centre Hospitalier Lyon-Sud, Pierre Bénite, France. 9. Department of Dermatology and Venereology, Medical University, Graz, Austria 10. Dermatology Unit, Second University of Naples, Naples, Italy. This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as Accepted doi: 10.1111/bjd.14045 This article is protected by copyright. All rights reserved. Please address all correspondence to: Aimilios Lallas, MD. -

The State of the Dysplastic Nevus in the 21St Century
The State of the Dysplastic Nevus in the 21st Century Keith Duffy, MD Associate professor Department of Dermatology University of Utah Department of Dermatology Disclosures • Myriad Genetics – Advisory board; honorarium • Castle Biosciences – Advisory board; honorarium What do I do? • Clinical – 60% Mohs micrographic and reconstructive surgery and high risk skin cancer – 40% Dermatopathology sign-out – Multidisciplinary cutaneous oncology program – Huntsman Cancer Institute • Administrative – Residency Program Director, Dermatology Department of Dermatology Department of Dermatology The current(ish) state of affairs… Do you believe dysplastic (Clark) nevi are truly premalignant lesions? 53% A. Yes B. No 25% C. Unsure 22% A. B. C. How do you report “dysplastic nevi”? A. Dysplastic nevus 62% B. Clark nevus C. Nevus with architectural disorder 19% D. Other 12% 7% A. B. C. D. Do you assign a histologic “grade” to these nevi? 87% A. Yes B. No 13% A. B. If yes, what grading system do you use? A. Cytology as three grades (mild, moderate, 73% severe) B. Cytology and architecture as two separate grades C. Cytology as two grades 8% 10% 10% only D. Other grading system A. B. C. D. Brief history • 1978 – Dr. Clark describes nevi associated with melanoma prone families – The B-K mole syndrome • 1978 – Dr. Lynch describes a single multi- generational family with melanoma and nevi – Familial atypical multiple mole melanoma syndrome (FAMMM) Brief history • 1980 – Dr. Elder and Clark describe ‘dysplastic nevi’ in a non-familial setting – Introduction of the term ‘dysplastic nevus syndrome’ • Familial and sporadic variants • Formally postulated that ‘dysplastic nevi’ are precursors of melanoma Dr. -

Lentigo Maligna Melanoma and Simulants Maui January 2020 Superficial Atypical Melanocytic Proliferations
Superficial Atypical Melanocytic Proliferations II. Lentigo Maligna Melanoma and Simulants Maui January 2020 Superficial Atypical Melanocytic Proliferations • RGP Melanomas • SSM, LMM, ALM, MLM • Intermediate lesions • Dysplastic nevi, Atypical lentiginous proliferations in high CSD skin; Atypical Acral lentiginous nevi • Superficial atypical melanocytic proliferations • Pagetoid plaque-like Spitz nevi; pigmented spindle cell nevus (Reed) • Special site nevi (genital, breast, scalp, ear, flexural, etc). • Superficial atypical melanocytic proliferations of uncertain significance • Atypical/unusual/uncertain examples of all of the above Superficial Atypical Melanocytic Proliferations • RGP Melanomas • SSM, LMM, ALM, MLM • Intermediate lesions • Dysplastic nevi, Atypical lentiginous proliferations in high CSD skin; Atypical Acral lentiginous nevi • Superficial atypical melanocytic proliferations • Pagetoid plaque-like Spitz nevi; pigmented spindle cell nevus (Reed) • Special site nevi (genital, breast, scalp, ear, flexural, etc). • Superficial atypical melanocytic proliferations of uncertain significance • Atypical/unusual/uncertain examples of all of the above High CSD Melanomas and Simulants. D Elder, Maui, HI Jan 2020 Lentigo maligna melanoma Atypical lentiginous nevi/proliferations High CSD: Lentiginous Nevi and Lentigo Maligna Melanoma and Simulant(s) • Lentiginous Melanoma of Sun-Damaged Skin • LMM in situ • LMM invasive • Distinction from Dysplastic Nevi (Dysplastic Nevus-like Melanoma/Nevoid Lentigo Maligna • Lentiginous Nevi of