A New Method for Vaccine Preservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children
March 19, 1999 / Vol. 48 / No. RR-2 Recommendations and Reports Rotavirus Vaccine for the Prevention of Rotavirus Gastroenteritis Among Children Recommendations of the Advisory Committee on Immunization Practices (ACIP) Continuing Education Examination Education Continuing Inside: U.S. DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Disease Control and Prevention (CDC) Atlanta, Georgia 30333 The MMWR series of publications is published by the Epidemiology Program Office, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30333. SUGGESTED CITATION Centers for Disease Control and Prevention. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1999;48(No. RR-2):[inclu- sive page numbers]. Centers for Disease Control and Prevention....................Jeffrey P. Koplan, M.D., M.P.H. Director The material in this report was prepared for publication by National Center for Infectious Diseases.................................. James M. Hughes, M.D. Director Division of Viral and Rickettsial Diseases ................. Brian W. J. Mahy, Ph.D., Sc.D. Director National Immunization Program ...........................................Walter A. Orenstein, M.D. Director Epidemiology and Surveillance Division ...........................John R. Livengood, M.D. Director The production of this report as an MMWR serial publication was coordinated in Epidemiology Program Office.................................... Stephen B. Thacker, M.D., M.Sc. Director Office of Scientific and Health Communications ......................John W. Ward, M.D. Director Editor, MMWR Series Recommendations and Reports................................... Suzanne M. Hewitt, M.P.A. Managing Editor Valerie R. Johnson Project Editor Morie M. Higgins Visual Information Specialist Vol. 48 / No. RR-2 MMWR i Contents Clinical and Epidemiologic Features of Rotavirus Disease............................................. -

Attenuation of Human Respiratory Syncytial Virus by Genome-Scale Codon-Pair Deoptimization
Attenuation of human respiratory syncytial virus by genome-scale codon-pair deoptimization Cyril Le Nouëna,1, Linda G. Brocka, Cindy Luongoa, Thomas McCartya, Lijuan Yanga, Masfique Mehedia, Eckard Wimmerb,1, Steffen Muellerb,2, Peter L. Collinsa, Ursula J. Buchholza,3, and Joshua M. DiNapolia,3,4 aRNA Viruses Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; and bDepartment of Molecular Genetics and Microbiology, Stony Brook University, Stony Brook, NY 11794 Contributed by Eckard Wimmer, June 18, 2014 (sent for review February 14, 2014) Human respiratory syncytial virus (RSV) is the most important viral acid coding is unaffected, CPD strains provide the same reper- agent of serious pediatric respiratory-tract disease worldwide. A toire of epitopes for inducing cellular and humoral immunity as vaccine or generally effective antiviral drug is not yet available. the WT pathogen. Recently, the CPD approach has been used We designed new live attenuated RSV vaccine candidates by successfully to attenuate poliovirus, influenza A virus, Strepto- codon-pair deoptimization (CPD). Specifically, viral ORFs were recoded coccus pneumonia, and HIV type 1 (5, 10–13). by rearranging existing synonymous codons to increase the content In the present work, four CPD RSV genomes were designed, of underrepresented codon pairs. Amino acid coding was com- synthesized, and recovered by reverse genetics. The CPD pletely unchanged. Four CPD RSV genomes were designed in recombinant (r) RSVs were attenuated and temperature- which the indicated ORFs were recoded: Min A (NS1, NS2, N, P, sensitive in vitro. Furthermore, we demonstrated that the CPD M, and SH), Min B (G and F), Min L (L), and Min FLC (all ORFs except rRSVs were attenuated and immunogenic in mice and African M2-1 and M2-2). -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful. -

Wastewater Based Epidemiology Perspective As a Faster Protocol for Detecting Coronavirus RNA in Human Populations: a Review with Specific Reference to SARS-Cov-2 Virus
pathogens Review Wastewater Based Epidemiology Perspective as a Faster Protocol for Detecting Coronavirus RNA in Human Populations: A Review with Specific Reference to SARS-CoV-2 Virus Milad Mousazadeh 1,2,†,‡ , Razieh Ashoori 3, Biswaranjan Paital 4 , I¸sıkKabda¸slı 5,‡ , Zacharias Frontistis 6 , Marjan Hashemi 7 , Miguel A. Sandoval 8,9,‡ , Samendra Sherchan 10, Kabita Das 11 and Mohammad Mahdi Emamjomeh 12,* 1 Student Research Committee, Qazvin University of Medical Sciences, Qazvin, Iran; [email protected] 2 Department of Environmental Health Engineering, School of Health, Qazvin University of Medical Sciences, Qazvin, Iran 3 Department of Environmental Health Engineering, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran; [email protected] 4 Redox Regulation Laboratory, College of Basic Science and Humanities, Odisha University of Agriculture and Technology, Bhubaneswar 751003, India; [email protected] 5 Environmental Engineering Department, Civil Engineering Faculty, Ayaza˘gaCampus, Istanbul˙ Technical University, Istanbul˙ 34469, Turkey; [email protected] 6 Department of Chemical Engineering, University of Western Macedonia, 50132 Kozani, Greece; Citation: Mousazadeh, M.; Ashoori, [email protected] 7 Environmental and Occupational Hazards Control Research Center, Shahid Beheshti University of Medical R.; Paital, B.; Kabda¸slı,I.; Frontistis, Sciences, Tehran, Iran; [email protected] Z.; Hashemi, M.; Sandoval, M.A.; 8 Laboratorio de Electroquímica Medio Ambiental LEQMA, Departamento -

Preventing Polio WHO Myanmar Newsletter Special , 5 August 2019
Preventing polio WHO Myanmar newsletter special , 5 August 2019 What is polio? Poliomyelitis (polio) is a highly infectious disease that is caused when the polio virus invades the nervous system of an infected person. Polio can cause paralysis and even death. Who is most at risk for polio? Poliovirus usually affects children under 5 years of age who are unvaccinated or under-vaccinated. The virus can also affect or be carried by adolescents and adults. photo credit: Ms Lei Lei Mon, WHO Myanmar The child or individual suspected of polio may A child receives oral polio vaccine at Thandaung township, complain of sudden onset of weakness of arms Kayin State, 21 July 2019 and/or legs. Oral polio vaccine is the vaccine Is there a cure for polio? recommended for polio eradication Can it be prevented? There is no cure for polio. The disease can severely paralyze, or even kill, an infected child. Is the polio vaccine safe? Oral polio vaccine is one of the safest vaccines Polio can be prevented by immunizing a child with ever developed. It is so safe it can be given to sick approrpiate vaccination. There are currently two children and newborns. Since 1961, when oral effective polio vaccines, the inactivated poliovirus polio vaccine was introduced, two billion children vaccine (IPV) and the live attenuated oral polio have been immunized against polio globally. vaccine (OPV). This is estimated to have saved at least 16 million children from permanent paralysis by polio, world- wide. It is also safe to administer multiple doses of polio vaccine to children. -

2021 Medicare Vaccine Coverage Part B Vs Part D
CDPHP® Medicare Advantage Vaccine Coverage Guide Part B (Medical) vs. Part D (Pharmacy) Medicare Part B (Medical): Medicare Part D (Pharmacy): Vaccinations or inoculations Vaccinations or inoculations are included when the administration is (except influenza, pneumococcal, reasonable and necessary for the prevention of illness. and hepatitis B for members at risk) are excluded unless they are directly related to the treatment of an injury or direct exposure to a disease or condition. • Influenza Vaccine (Flu) • BCG Vaccine • Pneumococcal Vaccine • Diphtheria/Tetanus/Acellular Pertussis Vaccine (ADACEL, (Pneumovax, Prevnar 13) BOOSTRIX, DAPTACEL, INFANRIX) • Hepatitis B Vaccine • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus (Recombivax, Engerix-B) Vaccine (KINRIX, QUADRACEL) for members at moderate • Diphtheria/Tetanus Vaccine (DT, Td, TDVAX, TENIVAC) to high risk • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus Vac • Other vaccines when directly cine/ Haemophilus Influenzae Type B Conjugate Vaccine (PENTACEL) related to the treatment of an • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus injury or direct exposure to a Vaccine/Hepatitis B Vaccine (PEDIARIX) disease or condition, such as: • Haemophilus Influenzae Type B Conjugate Vaccine (ActHIB, PedvaxHIB, • Antivenom Sera Hiberix) • Diphtheria/Tetanus Vaccine • Hepatitis A Vaccine, Inactivated (VAQTA) (DT, Td, TDVAX, TENIVAC) • Hepatitis B Vaccine, Recombinant (ENGERIX-B, RECOMBIVAX HB) • Rabies Virus Vaccine for members at low risk (RabAvert, -

IRES Knocks out Rabies
RESEARCH HIGHLIGHTS VIRAL PATHOGENESIS IRES knocks out rabies Non-segmented negative-strand from poliovirus or human rhinovirus Infection of neurons with RNA RNA viruses that can infect neurons type 2 (HRV2) enables downregula- viruses is initially controlled by in the central nervous system include tion of phosphoprotein expression interferon-β (IFNβ) and subsequently measles virus, mumps virus, Borna at the level of translation and can by virus-specific antibodies and virus, the emerging deadly Nipah render rabies virus avirulent. T-cell derived IFNβ. The rabies virus and Hendra viruses and rabies virus. Although rabies and other phosphoprotein, which is an essential Until now there has been no method non-segmented negative-strand cofactor of the viral polymerase and available to control the expression RNA viruses can replicate in all also has a role in encapsidation of of individual genes of these viruses. cell types, positive-strand RNA replicated genomes, functions to According to a report published in viruses, such as poliovirus and dampen the host response by coun- the Journal of Virology, replacing the HRV2, have a restricted host range. teracting the production of IFN. The transcription signals of the rabies There is evidence that IRESs in the most striking finding from this study virus phosphoprotein gene with 5′-untranslated regions of positive- is that translational attenuation of internal ribosome entry sites (IRESs) strand RNA viruses are subject to phosphoprotein through insertion of cell-type inhibition and can mediate IRESs from poliovirus or HRV2 lim- cell-type specificity. To test whether ited the ability of recombinant rabies Corbis IRESs can affect gene expression of viruses to counteract the production non-segmented negative-strand RNA of IFN, as shown by the failure of viruses, Marschalek et al. -
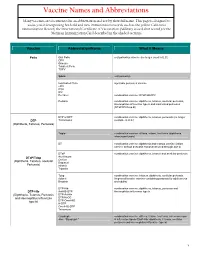
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Vaccine-Associated Paralytic Poliomyelitis and BCG-Osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome — Texas, 2013
Morbidity and Mortality Weekly Report Weekly / Vol. 63 / No. 33 August 22, 2014 Vaccine-Associated Paralytic Poliomyelitis and BCG-osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome — Texas, 2013 Robert Trimble, MD1, Jane Atkins, MD2, Troy C. Quigg, DO3, Cara C. Burns, PhD4, Gregory S. Wallace, MD4, Mary Thomas, MBBS5, Anil T. Mangla, PhD5, Anthony J. Infante, MD, PhD1 (Author affiliations at end of text) Poliovirus transmission has been eliminated in most of developed VAPP (2). A case of immunodeficiency-associated the world through the use of inactivated poliovirus vaccine vaccine-derived poliovirus (iVDPV) infection, without paraly- (IPV) and live, attenuated oral poliovirus vaccine (OPV). In sis, was diagnosed in an unvaccinated child with SCIDS in the United States, use of OPV was discontinued by the year 2005, but the source of the virus could not be definitively 2000 because of the potential for vaccine-associated paralytic identified (3). A woman in Minnesota aged 44 years with polio (VAPP); an average of eight cases were reported each long-standing common variable immunodeficiency died after year in the United States during 1980–2000 (1). Polio eradi- developing VAPP in 2009 (4). She was probably infected when cation efforts in other parts of the world continue to rely on her child received OPV approximately 12 years earlier. Case OPV to take advantage of transmission of poliovirus vaccine reports and cohort studies from several countries other than strains to unvaccinated persons in the population, lower cost, the United States demonstrate the continued occurrence of and ease of administration. In 2013, an infant aged 7 months iVDPVs and the need for ongoing surveillance (5). -

Chapter 18: Polio
Poliomyelitis Concepcion F. Estivariz, MD; Ruth Link-Gelles, PhD, MPH; and Tom Shimabukuro, MD, MPH, MBA Descriptions of polio-like illnesses have been around since antiquity, including a funerary stele depicting a man with Poliomyelitis a withered leg leaning on a staff. Michael Underwood first ● First described by Michael described a debility of the lower extremities in children that was Underwood in 1789 recognizable as poliomyelitis in England in 1789, but the disease ● Developed countries in was not observed in epidemics until the late 19th century. Northern Hemisphere During the first half of the 20th century, developed countries in suffered increasingly severe the Northern Hemisphere suffered epidemics each summer and epidemics in the first half fall that became increasingly severe. Polio infections peaked in of the 20th century the United States in 1952, with more than 21,000 paralytic cases. Following introduction of effective vaccines in 1955 (inactivated ● More than 21,000 paralytic polio vaccine, IPV) and 1961 (oral poliovirus vaccine, OPV), cases reported in the U.S. polio incidence declined rapidly. The last case of wild poliovirus in 1952 acquired in the United States was in 1979. ● Last case of wild poliovirus acquired in the U.S. was 1979 Poliovirus Poliovirus is a member of the enterovirus subgroup, family Picornaviridae. Picornaviruses are small, ether-insensitive viruses Poliovirus with an RNA genome. ● Enterovirus (RNA) ● Three serotypes: type 1, type 2, There are three poliovirus serotypes (type1, type 2, and type type 3 3); immunity to one serotype does not produce significant immunity to the other serotypes. ● Immunity to one serotype does not produce significant Poliovirus is rapidly inactivated by heat, formaldehyde, chlorine, immunity to other serotypes and ultraviolet light. -

Fact Sheet: Vaccine-Derived Poliovirus
FACT SHEET: VACCINE-DERIVED POLIOVIRUS Global efforts to immunise children with the oral polio vaccine (OPV) have reduced wild poliovirus cases by 99.9% since 1988, and of the three strains of wild poliovirus, only one remains in circulation – wild polio virus type 1 (WPV1). OPV is safe and effective and interrupts person-to-person spread of polio. However, on rare occasions, and only in under-immunised populations, the live weakened virus in OPV can circulate in a community for an extended period of time and mutate into a form that causes paralysis, known as circulating vaccine-derived poliovirus (cVDPV). cVDPVs are not related to, nor indicative of a re-emergence of wild poliovirus. cVDPVs have emerged as a key challenge in the final stage of eradication efforts; however, outbreaks can be stopped using the same proven tactics that stop wild poliovirus: strengthening polio surveillance systems and ensuring high vaccination coverage. For example, despite protracted conflict and instability, a 2017 cVDPV outbreak in Syria was successfully stopped in a matter of months by utilizing these tactics. If a population continues to be fully immunized, it will be protected against the spread of both wild and vaccine-derived polio. TYPES OF RISK TO STOP POLIOVIRUS DEFINITION FACTORS TRANSMISSION STRAINS Low immunisation Type 1: Causes 100% of current cases Infectious virus that invades Vaccinate all WILD rates, poor Type 2: Eradicated in 2015 the nervous system. Can cause children under POLIOVIRUS sanitation, high five years of age Type 3: Eradicated in 2019 (WPV) paralysis or death. population densities. with OPV. Rare, circulating virus mutated Low immunisation Vaccinate all CIRCULATING There are three types of cVDPV − from the weakened virus contained rates, poor children under five VACCINE-DERIVED types 1, 2 and 3, with type 2 currently POLIOVIRUS in OPV, which can only emerge in sanitation, high years of age with causing the vast majority of cases. -

Enteric Hepatitis Viruses1
Enteric hepatitis viruses1 Description The term “hepatitis viruses” refers to a diverse group of viruses all of which have the human liver as the primary target of replication and give rise to hepatitis or inflammation of the liver. Their replication may result in mass destruction of liver cells. Consequences include failure of the liver to fulfil basic functions such as removal of bilirubin from the circulatory blood system. Bilirubin is a red pigment released from red blood cells as they break down and are replaced by new cells. Excessive accumulation of the pigment in the bloodstream is manifest as yellow coloration of sites such as the eyes and the palms of the hands. This condition is known as jaundice and is also marked by dark urine and stools result- ing from the excretion of bilirubin. Another typical consequence of massive liver cell damage is release into the bloodstream of liver enzymes, including alanine transaminase (ALT) and aspartate transaminase (AST). Serum levels of these enzymes are used to diagnose hepatitis (Zuckerman & Thomas, 1993). Hepatitis may also be caused by other systemic pathogens such as cyto- megalovirus, yellow fever virus, and Leptospira bacteria, although the liver is not the primary or only target of these organisms. Liver cell damage and jaundice may also be caused by toxic compounds, including alcohol. Since the clinical symptoms caused by hepatitis viruses are very similar, and some of the viruses only emerged on a large scale in recent years, distinction of different aetiological agents has been progressively accomplished only since the 1960s. The first two hepatitis viruses that were distinguished were simply 1 This review was prepared by W.O.K.