Downloaded from Phosphositeplus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Gene Symbol Gene Description ACVR1B Activin a Receptor, Type IB
Table S1. Kinase clones included in human kinase cDNA library for yeast two-hybrid screening Gene Symbol Gene Description ACVR1B activin A receptor, type IB ADCK2 aarF domain containing kinase 2 ADCK4 aarF domain containing kinase 4 AGK multiple substrate lipid kinase;MULK AK1 adenylate kinase 1 AK3 adenylate kinase 3 like 1 AK3L1 adenylate kinase 3 ALDH18A1 aldehyde dehydrogenase 18 family, member A1;ALDH18A1 ALK anaplastic lymphoma kinase (Ki-1) ALPK1 alpha-kinase 1 ALPK2 alpha-kinase 2 AMHR2 anti-Mullerian hormone receptor, type II ARAF v-raf murine sarcoma 3611 viral oncogene homolog 1 ARSG arylsulfatase G;ARSG AURKB aurora kinase B AURKC aurora kinase C BCKDK branched chain alpha-ketoacid dehydrogenase kinase BMPR1A bone morphogenetic protein receptor, type IA BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase) BRAF v-raf murine sarcoma viral oncogene homolog B1 BRD3 bromodomain containing 3 BRD4 bromodomain containing 4 BTK Bruton agammaglobulinemia tyrosine kinase BUB1 BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) BUB1B BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) C9orf98 chromosome 9 open reading frame 98;C9orf98 CABC1 chaperone, ABC1 activity of bc1 complex like (S. pombe) CALM1 calmodulin 1 (phosphorylase kinase, delta) CALM2 calmodulin 2 (phosphorylase kinase, delta) CALM3 calmodulin 3 (phosphorylase kinase, delta) CAMK1 calcium/calmodulin-dependent protein kinase I CAMK2A calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha CAMK2B calcium/calmodulin-dependent -

Tumour-Agnostic Therapy for Pancreatic Cancer and Biliary Tract Cancer
diagnostics Review Tumour-Agnostic Therapy for Pancreatic Cancer and Biliary Tract Cancer Shunsuke Kato Department of Clinical Oncology, Juntendo University Graduate School of Medicine, 2-1-1, Hongo, Bunkyo-ku, Tokyo 113-8421, Japan; [email protected]; Tel.: +81-3-5802-1543 Abstract: The prognosis of patients with solid tumours has remarkably improved with the develop- ment of molecular-targeted drugs and immune checkpoint inhibitors. However, the improvements in the prognosis of pancreatic cancer and biliary tract cancer is delayed compared to other carcinomas, and the 5-year survival rates of distal-stage disease are approximately 10 and 20%, respectively. How- ever, a comprehensive analysis of tumour cells using The Cancer Genome Atlas (TCGA) project has led to the identification of various driver mutations. Evidently, few mutations exist across organs, and basket trials targeting driver mutations regardless of the primary organ are being actively conducted. Such basket trials not only focus on the gate keeper-type oncogene mutations, such as HER2 and BRAF, but also focus on the caretaker-type tumour suppressor genes, such as BRCA1/2, mismatch repair-related genes, which cause hereditary cancer syndrome. As oncogene panel testing is a vital approach in routine practice, clinicians should devise a strategy for improved understanding of the cancer genome. Here, the gene mutation profiles of pancreatic cancer and biliary tract cancer have been outlined and the current status of tumour-agnostic therapy in these cancers has been reported. Keywords: pancreatic cancer; biliary tract cancer; targeted therapy; solid tumours; driver mutations; agonist therapy Citation: Kato, S. Tumour-Agnostic Therapy for Pancreatic Cancer and 1. -

Developing Biomarkers for Livestock Science
Developing biomarkers for livestock Science Ongoing research and future developments Marinus te Pas Outline . Introduction ● What are biomarkers ● Why do we need them . Examples ● omics levels . The future ● Big data ● Systems biology / Synthetic biology 2 Introduction: What are biomarkers? . Biological processes underlie all livestock (production) traits ● Measure the status of a biological process = know the trait! . Can be any molecule in a cell ● No need to know the causal factor for a trait . Well known example: blood glucose level for diabetes Introduction: Why do we need biomarkers? . The mission of WageningenUR: Sustainably produce enough high quality food for all people on the planet with an ecological footprint as low as possible 4 What can the industry do with biomarkers? . Diagnostic tool ● What is the biological mechanism underlying a trait? . Prediction tool ● What outcome can I expect from an intervention? . Monitoring tool ● What is the actual status of a process? . Speed up your process, improve your traits Some examples * Transcriptomics * Proteomics * Metabolomics Why Biomarkers for meat quality? . Meat quality has low heritability (h2=0.1-0.2) ● Predictive capacity of genetic markers low . High environmental influence ● Feed, animal handling (stress), management (housing), ... Meat quality can only be measured after 1-several days post slaughter . Need to differentiate between retail, processing industry, restaurants, .... Biomarkers can do all that and more faster, predictive, .. Example Transcriptomics biomarkers for meat quality . Pork production chain . Biomarkers for traits . High quality fresh pork . Meat colour N production chain ● A* 14 . German Pietrain ● L* 4 (microarray) ● Reflection 10 . Verification: Danish . Drip loss 2 Yorkshire (PCR) . Ultimate pH 6 . -

Application of a MYC Degradation
SCIENCE SIGNALING | RESEARCH ARTICLE CANCER Copyright © 2019 The Authors, some rights reserved; Application of a MYC degradation screen identifies exclusive licensee American Association sensitivity to CDK9 inhibitors in KRAS-mutant for the Advancement of Science. No claim pancreatic cancer to original U.S. Devon R. Blake1, Angelina V. Vaseva2, Richard G. Hodge2, McKenzie P. Kline3, Thomas S. K. Gilbert1,4, Government Works Vikas Tyagi5, Daowei Huang5, Gabrielle C. Whiten5, Jacob E. Larson5, Xiaodong Wang2,5, Kenneth H. Pearce5, Laura E. Herring1,4, Lee M. Graves1,2,4, Stephen V. Frye2,5, Michael J. Emanuele1,2, Adrienne D. Cox1,2,6, Channing J. Der1,2* Stabilization of the MYC oncoprotein by KRAS signaling critically promotes the growth of pancreatic ductal adeno- carcinoma (PDAC). Thus, understanding how MYC protein stability is regulated may lead to effective therapies. Here, we used a previously developed, flow cytometry–based assay that screened a library of >800 protein kinase inhibitors and identified compounds that promoted either the stability or degradation of MYC in a KRAS-mutant PDAC cell line. We validated compounds that stabilized or destabilized MYC and then focused on one compound, Downloaded from UNC10112785, that induced the substantial loss of MYC protein in both two-dimensional (2D) and 3D cell cultures. We determined that this compound is a potent CDK9 inhibitor with a previously uncharacterized scaffold, caused MYC loss through both transcriptional and posttranslational mechanisms, and suppresses PDAC anchorage- dependent and anchorage-independent growth. We discovered that CDK9 enhanced MYC protein stability 62 through a previously unknown, KRAS-independent mechanism involving direct phosphorylation of MYC at Ser . -

DNAJB1–PRKACA Fusion Kinase Interacts with Β-Catenin and the Liver
DNAJB1–PRKACA fusion kinase interacts with INAUGURAL ARTICLE β-catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma Edward R. Kastenhubera,b, Gadi Lalazarc, Shauna L. Houlihana, Darjus F. Tschaharganehd,e, Timour Baslana, Chi-Chao Chena, David Requenac, Sha Tiana, Benedikt Bosbachf, John E. Wilkinsong, Sanford M. Simonc, and Scott W. Lowea,h,1 aDepartment of Cancer Biology and Genetics, Memorial Sloan Kettering Cancer Center, New York, NY 10065; bLouis V. Gerstner Jr. Graduate School of Biomedical Sciences, Memorial Sloan Kettering Cancer Center, New York, NY 10065; cLaboratory of Cellular Biophysics, The Rockefeller University, New York, NY 10065; dHelmholtz University Group “Cell Plasticity and Epigenetic Remodeling,” German Cancer Research Center (DKFZ), 69120 Heidelberg, Germany; eInstitute of Pathology, University Hospital, 69120 Heidelberg, Germany; fOncology Target Discovery Program, Pfizer Inc., Pearl River, NY 10965; gDepartment of Pathology, University of Michigan School of Medicine, Ann Arbor, MI 48109; and hHoward Hughes Medical Institute, New York, NY 10065 This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2017. Contributed by Scott W. Lowe, October 26, 2017 (sent for review September 22, 2017; reviewed by Nabeel M. Bardeesy and David A. Largaespada) A segmental deletion resulting in DNAJB1–PRKACA gene fusion is any known etiological risk factors such as alcoholism, chronic hep- now recognized as the signature genetic event of fibrolamellar hepa- atitis infection, or liver flukes (8). tocellular carcinoma (FL-HCC), a rare but lethal liver cancer that pri- Currently, FL-HCC is diagnosed on the basis of histological marily affects adolescents and young adults. -

Epigenetic Loss of the RNA Decapping Enzyme NUDT16 Mediates C-MYC Activation in T-Cell Acute Lymphoblastic Leukemia
OPEN Leukemia (2017) 31, 1622–1657 www.nature.com/leu LETTERS TO THE EDITOR Epigenetic loss of the RNA decapping enzyme NUDT16 mediates C-MYC activation in T-cell acute lymphoblastic leukemia Leukemia (2017) 31, 1622–1625; doi:10.1038/leu.2017.99 Having found the aforementioned NUDT16 CpG island methy- lation profiles, we studied in greater detail their association with the possible transcriptional inactivation of the NUDT16 gene at the RNA and protein levels in leukemia cell lines. We first It is possible that the occurrence of intrinsic defects in RNA performed bisulfite genomic sequencing of mutiple clones in the – processing pathways, such as RNA decapping,1 3 contribute to the T-cell Acute Lymphoblastic Leukemia (T-ALL) cell lines CCRF-CEM, distorted RNA landscapes of cancer cells. After transcription by Jurkat, MOLT-4 and MOLT-16 using primers that encompassed the RNA polymerase II, RNA molecules are equipped with a 5´-end transcription start site-associated CpG island and confirmed the N7-methyl guanosine (m7G)-cap. This m7G-cap is essential for hypermethylated status of the 5′-end region of NUDT16 in translation, stabilizing the RNA molecule and protecting it from comparison to normal T lymphocytes (Figure 1b), validating the – exonucleolytic breakdown.1 3 For RNA decay to occur the DNA methylation patterns obtained by the microarray approach m7G-cap first needs to be removed. This process is known as (Supplementary Figure S2). In contrast, normal T lymphocytes, the – decapping.1 3 The decapping mRNA 2 (DCP2) enzyme,4 also T-ALL cell lines KOPN-8, REH and RS4;11 and the leukemia cell known as the nucleoside diphosphate-linked moiety X motif lines HL-60 and K562 derived from myeloid lineage were all found 20 (NUDT20), was originally thought to be the only mammalian to be unmethylated (Figure 1b; Supplementary Figure S2). -

Supplementary Table 1
SI Table S1. Broad protein kinase selectivity for PF-2771. Kinase, PF-2771 % Inhibition at 10 μM Service Kinase, PF-2771 % Inhibition at 1 μM Service rat RPS6KA1 (RSK1) 39 Dundee AURKA (AURA) 24 Invitrogen IKBKB (IKKb) 26 Dundee CDK2 /CyclinA 21 Invitrogen mouse LCK 25 Dundee rabbit MAP2K1 (MEK1) 19 Dundee AKT1 (AKT) 21 Dundee IKBKB (IKKb) 16 Dundee CAMK1 (CaMK1a) 19 Dundee PKN2 (PRK2) 14 Dundee RPS6KA5 (MSK1) 18 Dundee MAPKAPK5 14 Dundee PRKD1 (PKD1) 13 Dundee PIM3 12 Dundee MKNK2 (MNK2) 12 Dundee PRKD1 (PKD1) 12 Dundee MARK3 10 Dundee NTRK1 (TRKA) 12 Invitrogen SRPK1 9 Dundee MAPK12 (p38g) 11 Dundee MAPKAPK5 9 Dundee MAPK8 (JNK1a) 11 Dundee MAPK13 (p38d) 8 Dundee rat PRKAA2 (AMPKa2) 11 Dundee AURKB (AURB) 5 Dundee NEK2 11 Invitrogen CSK 5 Dundee CHEK2 (CHK2) 11 Invitrogen EEF2K (EEF-2 kinase) 4 Dundee MAPK9 (JNK2) 9 Dundee PRKCA (PKCa) 4 Dundee rat RPS6KA1 (RSK1) 8 Dundee rat PRKAA2 (AMPKa2) 4 Dundee DYRK2 7 Dundee rat CSNK1D (CKId) 3 Dundee AKT1 (AKT) 7 Dundee LYN 3 BioPrint PIM2 7 Invitrogen CSNK2A1 (CKIIa) 3 Dundee MAPK15 (ERK7) 6 Dundee CAMKK2 (CAMKKB) 1 Dundee mouse LCK 5 Dundee PIM3 1 Dundee PDPK1 (PDK1) (directed 5 Invitrogen rat DYRK1A (MNB) 1 Dundee RPS6KB1 (p70S6K) 5 Dundee PBK 0 Dundee CSNK2A1 (CKIIa) 4 Dundee PIM1 -1 Dundee CAMKK2 (CAMKKB) 4 Dundee DYRK2 -2 Dundee SRC 4 Invitrogen MAPK12 (p38g) -2 Dundee MYLK2 (MLCK_sk) 3 Invitrogen NEK6 -3 Dundee MKNK2 (MNK2) 2 Dundee RPS6KB1 (p70S6K) -3 Dundee SRPK1 2 Dundee AKT2 -3 Dundee MKNK1 (MNK1) 2 Dundee RPS6KA3 (RSK2) -3 Dundee CHEK1 (CHK1) 2 Invitrogen rabbit MAP2K1 (MEK1) -4 Dundee -

An Extensive Program of Periodic Alternative Splicing Linked to Cell
RESEARCH ARTICLE An extensive program of periodic alternative splicing linked to cell cycle progression Daniel Dominguez1,2, Yi-Hsuan Tsai1,3, Robert Weatheritt4, Yang Wang1,2, Benjamin J Blencowe4*, Zefeng Wang1,5* 1Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, United States; 2Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, United States; 3Program in Bioinformatics and Computational Biology, University of North Carolina at Chapel Hill, Chapel Hill, United States; 4Donnelly Centre and Department of Molecular Genetics, University of Toronto, Toronto, Canada; 5Key Lab of Computational Biology, CAS-MPG Partner Institute for Computational Biology, Chinese Academy of Science, Shanghai, China Abstract Progression through the mitotic cell cycle requires periodic regulation of gene function at the levels of transcription, translation, protein-protein interactions, post-translational modification and degradation. However, the role of alternative splicing (AS) in the temporal control of cell cycle is not well understood. By sequencing the human transcriptome through two continuous cell cycles, we identify ~ 1300 genes with cell cycle-dependent AS changes. These genes are significantly enriched in functions linked to cell cycle control, yet they do not significantly overlap genes subject to periodic changes in steady-state transcript levels. Many of the periodically spliced genes are controlled by the SR protein kinase CLK1, whose level undergoes cell cycle- dependent fluctuations via an auto-inhibitory circuit. Disruption of CLK1 causes pleiotropic cell cycle defects and loss of proliferation, whereas CLK1 over-expression is associated with various *For correspondence: cancers. These results thus reveal a large program of CLK1-regulated periodic AS intimately [email protected] (BJB); associated with cell cycle control. -
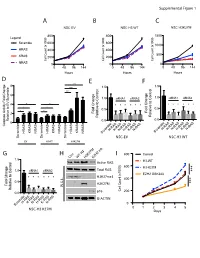
C a B D G E F
Supplemental Figure 1 A B C NSC EV NSC H3 WT NSC H3K27M 800 800 1500 ) ) Legend ) Scramble 600 600 x1000 x1000 x1000 ( ( ( 1000 t t t n n HRAS n 400 400 u u u o o o C C C 500 l l KRAS l 200 200 el el el C C C NRAS 0 0 0 0 48 96 144 0 48 96 144 0 48 96 144 Hours Hours Hours D *** E F 8 1.5 1.5 e *** l l *** ro ro t 6 t 1.0 siRNA1 siRNA2 1.0 siRNA1 siRNA2 Con Con o o t 4 * * t * * * * * * e * * * * * * e v i * * v i t 0.5 * * t 0.5 Fold Change 2 Fold Change Rela Rela Relative to EV Scrambl 0.0 0.0 0 Caspase Activity Fold Change H-RASK-RASN-RASH-RASK-RASN-RAS H-RASK-RASN-RASH-RASK-RASN-RAS KRAS KRAS KRAS HRAS NRAS HRAS NRAS HRAS NRAS Scramble Scramble Scramble Scramble Scramble NSC-EV NSC-H3 WT EV H3WT H3K27M G H I 800 Control Con WT H3 H3K27MEZH2 inb 1.5 l H3-WT Active RAS ro ) t 600 H3-K27M 1.0 siRNA1 siRNA2 Total RAS *** Con x1000 ( o EZH2 GSK343 t t * * * * * * H3K27me3 n e 400 u v i o t 0.5 *** H3K27M C Fold Change l W.C.L. el Rela p16 C 200 0.0 B-ACTIN H-RASK-RASN-RASH-RASK-RASN-RAS 0 Scramble NSC-H3 K27M 0 1 2 3 4 5 Days E C A 100 Cell Viability 100 25 50 75 Cell Viability 25 50 75 0 0 Fold Change MOCK MOCK Relative to Control Scramble * 0.0 0.2 0.4 0.6 0.8 1.0 1.2 MYC Scramble MYC MYC * PDGFRA Scramble siRNA PDGFRA MYC PDGFRA AURKA AURKA DIPG007siRNA2 PDGFRA NSC H3K27MsiRNA1and AURKA LAMTOR3 AURKA LAMTOR3 LAMTOR3 LIN28A LAMTOR3 LIN28A LIN28B LIN28A NSC-EV LIN28A MAP2K3 LIN28B LIN28B LIN28B MAP2K5 siRNA1 MAP2K3 MAP2K3 MAP3K2 MAP2K3 MAP3K7 MAP2K5 MAP2K5 MAPK7 MAP2K5 2 MAP3K2 siRNA1 siRNA2 MAP3K2 ZAK MAP3K7 MAP3K2 MAP3K7 MAPK7 MAP3K7 -

P-MYLK (Tyr 464)-R: Sc-17182-R
SAN TA C RUZ BI OTEC HNOL OG Y, INC . p-MYLK (Tyr 464)-R: sc-17182-R BACKGROUND SOURCE The Ca 2+ /calmodulin-dependent protein kinases (CaM kinases) are a struc - p-MYLK (Tyr 464)-R is a rabbit polyclonal antibody raised against a short turally related subfamily of serine/threonine kinases that includes CaMKI, amino acid sequence containing phosphorylated Tyr 464 of MYLK of human CaMKII, CaMKIV and myosin light chain kinases (MYLK, also designated origin. MLCK). The MYLK kinases phosphorylate myosin regulatory light chains to catalyze myosin interaction with actin filaments resulting in contractile activi - PRODUCT ty. Non-muscle, smooth muscle and skeletal/cardiac muscle MYLK isoforms Each vial contains 200 µg IgG in 1.0 ml of PBS with < 0.1% sodium azide exist. The MYLK gene (also designated MYLK1) encodes both smooth mus - and 0.1% gelatin. cle and non-muscle isoforms as well as telokin, a small C-terminal isoform Blocking peptide available for competition studies, sc-17182 P, (100 µg expressed only in smooth muscle with the capacity to stabilize unphosphor- pep tide in 0.5 ml PBS containing < 0.1% sodium azide and 0.2% BSA). ylated myosin filaments. Multiple transcript variants are described for the MYLK gene. Smooth-muscle and non-muscle MYLK isoforms are expressed APPLICATIONS in a wide variety of adult and fetal tissues. The skeletal/cardiac muscle iso - forms of MYLK are encoded by a separate gene, MYLK2 (also designated p-MYLK (Tyr 464)-R is recommended for detection of Tyr 464 phosphorylated skMLCK). MYLK appears to be a target for PAKs (p21-activated kinases). -

GAK and PRKCD Are Positive Regulators of PRKN-Independent
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.05.369496; this version posted November 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 GAK and PRKCD are positive regulators of PRKN-independent 2 mitophagy 3 Michael J. Munson1,2*, Benan J. Mathai1,2, Laura Trachsel1,2, Matthew Yoke Wui Ng1,2, Laura 4 Rodriguez de la Ballina1,2, Sebastian W. Schultz2,3, Yahyah Aman4, Alf H. Lystad1,2, Sakshi 5 Singh1,2, Sachin Singh 2,3, Jørgen Wesche2,3, Evandro F. Fang4, Anne Simonsen1,2* 6 1Division of Biochemistry, Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo 7 2Centre for Cancer Cell Reprogramming, Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, N-0316, Oslo, Norway. 8 3Department of Molecular Cell Biology, The Norwegian Radium Hospital Montebello, N-0379, Oslo, Norway 9 4Department of Clinical Molecular Biology, University of Oslo and Akershus University Hospital, 1478 Lørenskog, Norway 10 11 Keywords: GAK, Cyclin G Associated Kinase, PRKCD, Protein Kinase C Delta, Mitophagy, DFP, 12 DMOG, PRKN 13 14 *Corresponding Authors: 15 [email protected] 16 [email protected] 17 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.05.369496; this version posted November 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.