Ex Vivo Generation and Characterization of Human Hyaline and Elastic Cartilaginous Microtissues for Tissue Engineering Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Applications of Chondrocyte-Based Cartilage Engineering: an Overview
Hindawi Publishing Corporation BioMed Research International Volume 2016, Article ID 1879837, 17 pages http://dx.doi.org/10.1155/2016/1879837 Review Article Applications of Chondrocyte-Based Cartilage Engineering: An Overview Abdul-Rehman Phull,1 Seong-Hui Eo,1 Qamar Abbas,1 Madiha Ahmed,2 and Song Ja Kim1 1 Department of Biological Sciences, College of Natural Sciences, Kongju National University, Gongjudaehakro 56, Gongju 32588, Republic of Korea 2Department of Pharmacy, Quaid-i-Azam University, Islamabad 45320, Pakistan Correspondence should be addressed to Song Ja Kim; [email protected] Received 14 May 2016; Revised 24 June 2016; Accepted 26 June 2016 Academic Editor: Magali Cucchiarini Copyright © 2016 Abdul-Rehman Phull et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Chondrocytes are the exclusive cells residing in cartilage and maintain the functionality of cartilage tissue. Series of biocomponents such as different growth factors, cytokines, and transcriptional factors regulate the mesenchymal stem cells (MSCs) differentiation to chondrocytes. The number of chondrocytes and dedifferentiation are the key limitations in subsequent clinical application of the chondrocytes. Different culture methods are being developed to overcome such issues. Using tissue engineering and cell based approaches, chondrocytes offer prominent therapeutic option specifically in orthopedics for cartilage repair and to treat ailments such as tracheal defects, facial reconstruction, and urinary incontinence. Matrix-assisted autologous chondrocyte transplantation/implantation is an improved version of traditional autologous chondrocyte transplantation (ACT) method. An increasing number of studies show the clinical significance of this technique for the chondral lesions treatment. -

Bone & Cartilage
Compiled and circulated by Mr. Suman Kalyan Khanra, SACT, Dept. of Physiology, Narajole Raj college BONE & CARTILAGE (Structure, Function, Classification of BONE & CARTILAGE) BY Suman Kalyan Khanra SACT Department of Physiology NARAJOLE RAJ COLLEGE Narajole; Paschim Medinipur 1 | P a g e ZOOLOGY: SEM-III, Paper-C6T: Animal Physiology, Unit-2: Bone & Cartilage Compiled and circulated by Mr. Suman Kalyan Khanra, SACT, Dept. of Physiology, Narajole Raj college BONE Definition: A bone is a somatic structure that is comprised of calcified connective tissue. Ground substance and collagen fibers create a matrix that contains osteocytes. These cells are the most common cell found in mature bone and responsible for maintaining bone growth and density. Within the bone matrix both calcium and phosphate are abundantly stored, strengthening and densifying the structure. Each bone is connected with one or more bones and are united via a joint (only exception: hyoid bone). With the attached tendons and musculature, the skeleton acts as a lever that drives the force of movement. The inner core of bones (medulla) contains either red bone marrow (primary site of hematopoiesis) or is filled with yellow bone marrow filled with adipose tissue. The main outcomes of bone development are endochondral and membranous forms. This particular characteristic along with the general shape of the bone are used to classify the skeletal system. The main shapes that are recognized include: long short flat sesamoid irregular Types of bone Long bones These bones develop via endochondral ossification, a process in which the hyaline cartilage plate is slowly replaced. A shaft, or diaphysis, connects the two ends known as the epiphyses (plural for epiphysis). -

Histologia Animal
Índex de termes castellans Índex de termes anglesos ácido hialurónico, 1 eosinófi lo, 38 líquido cerebroespinal, 73 proteína estructural, 115 adhesive protein, 114 ectodermic, 33 leucocyte, 59 oogenesis, 105 adipocito, 2 epitelio, 39 macrófago, 74 proteoglucano, 116 adipose tissue, 129 elastic cartilage, 15 leukocyte, 59 osseous tissue, 134 agranulocito, 3 epitelio estratifi cado, 40 mastocito, 75 receptor, 117 adypocite, 2 elastin, 34 lymphocyte, 71 ossifi cation, 107 amielínico –ca, 4 epitelio seudoestratifi cado, 41 matriz extracelular, 76 retículo sarcoplasmático, 118 agranulocyte, 3 electrical synapse, 123 lymphocytopoiesis, 72 osteoblast, 108 amígdala, 5 epitelio simple, 42 medula, 77 sangre, 119 amyelinic, 4 endochondral, 35 lymphoid organs, 106 osteoclast, 110 anticuerpo, 6 eritrocito, 43 médula, 77 sarcómero, 120 amygdala, 5 endocrine gland, 55 lymphopoiesis, 72 osteocyte, 109 APC, 23 eritropoyesis, 44 médula ósea amarilla, 78 sinapsis, 122 animal histology, 66 endoderm, 36 macrophage, 74 peripheral nervous system, 127 axón, 7 espermatogénesis, 45 médula ósea roja, 79 sinapsis eléctrica, 123 antibody, 6 endodermal, 37 marrow, 77 plasma, 112 barrera hematoencefálica, 8 estereocilio, 46 megacariocito, 80 sinapsis química, 124 antigen-presenting cell, 23 endodermic, 37 mast cell, 75 plasma cell, 22 basófi lo –la, 9 fecundación, 47 megacariocitopoyesis, 81 sistema inmunitario, 125 APC, 23 eosinophil, 38 mastocyte, 75 plasmacyte, 22 bazo, 82 fi bra muscular, 48 memoria inmunitaria, 83 sistema nervioso central, 126 axon, 7 eosinophile, 38 -

Differentiation of the Secondary Elastic Cartilage in the External Ear of the Rat
Int..I. Dc\'. Bi,,!. 35: 311-320 (1991) 311 Differentiation of the secondary elastic cartilage in the external ear of the rat ZELIMIR BRADAMANTE*', LJILJANA KOSTOVIC-KNEZEVIC', BOZICA LEVAK-SVAJGER' and ANTON SVAJGER' 'Institute of Histology and Embryology and 2/nstitute of Biology, Faculty of Medicine, University of Zagreb, Republic of Croatia, Yugoslavia ABSTRACT The cartilage in the external ear of the rat belongs to the group of secondary cartilages and it has a unique structural organization. The chondrocytes aretransformed intotypical adipose cells, the proteoglycan cartilage matrix is reduced to thin capsules around the cells and the rest of the extracelullar matrix is occupied by a network of coarse elastic fibers. It appears late in development 116-day fetus) and needs more than one month for final development. The differentiation proceeds in several steps which partly overlap: the appearance of collagen fibrils, elastin fibers, the proteoglycan matrix, and the adipose transformation of chondrocytes. The phenotype of this cartilage and the course of its differentiation are very stable, even in very atypical experimental environmental conditions. The only exceptions are explants in organ culture in vitro and perichondrial regenerates. In these conditions the development of elastic fibers is slow and poor while the production of the proteogycan matrix is abundant. The resulting cartilage then displays structural characteristics of hyaline cartilage rather than those of the initial elastic one. KEY WORDS: elastic mrlilagf, tI/(Wdrogfllt'.\'i.\. plasfogellf'.\is, extenuil Ntr, rat Introduction Structural organization The elastic cartilage belongs to the group of secondary or ac- According to Baecker (1928) and Schaffer (1930) the cartilage cessory cartilages since it is not a part of the cartilagineous in the external ear (external auditory meatus and pinna) of the rat primordium of the body skeleton as is most hyaline cartilage can be defined as: a) cellular or parenchymatous, [jecause of the (Schaffer, 1930). -

16 Cartilage
Cartilage Cartilage serves as a rigid yet lightweight and flexible supporting tissue. It forms the framework for the respiratory passages to prevent their collapse, provides smooth "bearings" at joints, and forms a cushion between the vertebrae, acting as a shock absorber for the spine. Cartilage is important in determining the size and shape of bones and provides the growing areas in many bones. Its capacity for rapid growth while maintaining stiffness makes cartilage suitable for the embryonic skeleton. About 75% of the water in cartilage is bound to proteoglycans, and these compounds are important in the transport of fluids, electrolytes, and nutrients throughout the cartilage matrix. Although adapted to provide support, cartilage contains only the usual elements of connective tissue cells, fibers, and ground substance. It is the ground substance that gives cartilage its firm consistency and ability to withstand compression and shearing forces. Collagen and elastic fibers embedded in the ground substance impart tensile strength and elasticity. Together, the fibers and ground substance form the matrix of cartilage. Cartilage differs from other connective tissues in that it lacks nerves, blood and lymphatic vessels and is nourished entirely by diffusion of materials from blood vessels in adjacent tissues. Although relatively rigid, the cartilage matrix has high water content and is freely permeable, even to fairly large particles. Classification of cartilage into hyaline, elastic, and fibrous types is based on differences in the abundance and type of fibers in the matrix. Hyaline Cartilage Hyaline cartilage is the most common type of cartilage and forms the costal cartilages, articular cartilages of joints, and cartilages of the nose, larynx, trachea, and bronchi. -

Design and Fabrication of Nonconventional Optical Components by Precision Glass Molding
Design and Fabrication of Nonconventional Optical Components by Precision Glass Molding DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Peng He Graduate Program in Industrial and Systems Engineering The Ohio State University 2014 Dissertation Committee: Dr. Allen Y. Yi, Advisor Dr. Jose M. Castro Dr. L. James Lee Copyright by Peng He 2014 Abstract Precision glass molding is a net-shaping process to fabricate glass optics by replicating optical features from precision molds to glass at elevated temperature. The advantages of precision glass molding over traditional glass lens fabrication methods make it especially suitable for the production of optical components with complicated geometries, such as aspherical lenses, diffractive hybrid lenses, microlens arrays, etc. Despite of these advantages, a number of problems must be solved before this process can be used in industrial applications. The primary goal of this research is to determine the feasibility and performance of nonconventional optical components formed by precision glass molding. This research aimed to investigate glass molding by combing experiments and finite element method (FEM) based numerical simulations. The first step was to develop an integrated compensation solution for both surface deviation and refractive index drop of glass optics. An FEM simulation based on Tool-Narayanaswamy-Moynihan (TNM) model was applied to predict index drop of the molded optical glass. The predicted index value was then used to compensate for the optical design of the lens. Using commercially available general purpose software, ABAQUS, the entire process of glass molding was simulated to calculate the surface deviation from the adjusted lens geometry, which was applied to final mold shape modification. -

Cartilage & Bone
Cartilage & bone Red: important. Black: in male|female slides. Gray: notes|extra. Editing File ➢ OBJECTIVES • describe the microscopic structure, distribution and growth of the different types of Cartilage • describe the microscopic structure, distribution and growth of the different types of Bone Histology team 437 | MSK block | Lecture one ➢ REMEMBER from last block (connective tissue lecture) Components of connective tissue Fibers Cells Collagenous, Matrix difference elastic & the intercellular substance, in which types reticular cells and fibers are embedded. Rigid (rubbery, Hard (solid) Fluid (liquid) Soft firm) “Cartilage” “Bone” “Blood” “C.T Proper” Histology team 437 | MSK block | Lecture one CARTILAGE “Chondro- = relating to cartilage” BONE “Osteo- = relating to bone” o Its specialized type of connective tissue with a rigid o Its specialized type of connective tissue with a hard matrix (ﻻ يكسر بسهولة) matrix o Its usually nonvascular (avascular = lack of blood o Types: vessels) 1) Compact bone 2) Spongy bone o Its poor nerve supply o Components: 1) Bone cells: o All cartilage contain collagen fiber type II • Osteogenic cells • Osteoblasts o Types: • Osteocytes 1) Hyaline cartilage (main type) • Osteoclasts 2) Elastic cartilage 2) Bone Matrix (calcified osteoid tissue): 3) Fibrocartilage • hard because it is calcified (Calcium salts) • It contains collagen fibers type I • It forms bone lamellae and trabeculae 3) Periosteum 4) Endosteum o Functions: 1) body support 2) protection of vital organs as brain & bone marrow 3) calcium -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -
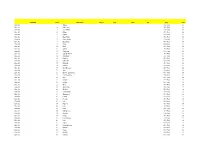
Set Name Card Description Sketch Auto
Set Name Card Description Sketch Auto Mem #'d Odds Point Base Set 1 Angela 4 Per Pack 24 Base Set 2 Anti-Venom 4 Per Pack 24 Base Set 3 Doc Samson 4 Per Pack 24 Base Set 4 Attuma 4 Per Pack 24 Base Set 5 Bedlam 4 Per Pack 24 Base Set 6 Black Knight 4 Per Pack 24 Base Set 7 Black Panther 4 Per Pack 24 Base Set 8 Black Swan 4 Per Pack 24 Base Set 9 Blade 4 Per Pack 24 Base Set 10 Blink 4 Per Pack 24 Base Set 11 Callisto 4 Per Pack 24 Base Set 12 Cannonball 4 Per Pack 24 Base Set 13 Captain Universe 4 Per Pack 24 Base Set 14 Challenger 4 Per Pack 24 Base Set 15 Punisher 4 Per Pack 24 Base Set 16 Dark Beast 4 Per Pack 24 Base Set 17 Darkhawk 4 Per Pack 24 Base Set 18 Collector 4 Per Pack 24 Base Set 19 Devil Dinosaur 4 Per Pack 24 Base Set 20 Ares 4 Per Pack 24 Base Set 21 Ego The Living Planet 4 Per Pack 24 Base Set 22 Elsa Bloodstone 4 Per Pack 24 Base Set 23 Eros 4 Per Pack 24 Base Set 24 Fantomex 4 Per Pack 24 Base Set 25 Firestar 4 Per Pack 24 Base Set 26 Ghost 4 Per Pack 24 Base Set 27 Ghost Rider 4 Per Pack 24 Base Set 28 Gladiator 4 Per Pack 24 Base Set 29 Goblin Knight 4 Per Pack 24 Base Set 30 Grandmaster 4 Per Pack 24 Base Set 31 Hazmat 4 Per Pack 24 Base Set 32 Hercules 4 Per Pack 24 Base Set 33 Hulk 4 Per Pack 24 Base Set 34 Hyperion 4 Per Pack 24 Base Set 35 Ikari 4 Per Pack 24 Base Set 36 Ikaris 4 Per Pack 24 Base Set 37 In-Betweener 4 Per Pack 24 Base Set 38 Khonshu 4 Per Pack 24 Base Set 39 Korvus 4 Per Pack 24 Base Set 40 Lady Bullseye 4 Per Pack 24 Base Set 41 Lash 4 Per Pack 24 Base Set 42 Legion 4 Per Pack 24 Base Set 43 Living Lightning 4 Per Pack 24 Base Set 44 Maestro 4 Per Pack 24 Base Set 45 Magus 4 Per Pack 24 Base Set 46 Malekith 4 Per Pack 24 Base Set 47 Manifold 4 Per Pack 24 Base Set 48 Master Mold 4 Per Pack 24 Base Set 49 Metalhead 4 Per Pack 24 Base Set 50 M.O.D.O.K. -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28 -

Tooling & Molds User Guide
TOOLING & MOLDS USER GUIDE WHERE GREAT IDEAS TAKE SHAPE www.generalplastics.com IMPORTANT: BEFORE YOU START, TEST YOUR MATERIALS Select the material you plan to use for testing, and then test the tooling material under the expected process conditions to ensure it is suitable and stable. This is recommended to ensure good tooling performance, and it should be performed as part of your process before you commit to a larger program. All General Plastics material products are manufactured in the United States and are free of CFCs and VOCs. Customer Service and Product Experts 1-800-806-6051 or 1-253-473-5000 Monday-Friday 6:30am-5pm, Pacific Time LAST-A-FOAM® products mentioned in this guide include: FR-3700, FR-4500, FR-4700, FR-4800, and FR-7100. INTRODUCTION TO THE USER GUIDE General Plastics Mtg. Co. prepared this guide to assist you Our knowledgeable customer service team and product with recommendations, general guidelines and a reference experts are ready and available to answer questions you to address common applications using the LAST-A-FOAM® may have and to help you attain the best possible results high-density, rigid polyurethane foam product line. using our products. We can provide recommendations on product selection, design, use, and testing services or Here you will find information on material properties and product literature. performance, application considerations, and helpful tips and resources when using our products. Specific to this guide are the FR-7100 Multi-Use Core and Modeling Board Series, the FR-4800 High Temperature Tooling Board, the FR-4700 High-Temperature Tooling Board Series, the FR-4500 Tooling Board Series, and the FR-3700 Performance Core Series. -

Institution Spons Agency Available from Abstract
DOCUMENT RESUME ED 422 492 CE 077 015 TITLE Mold Making Series. Educational Resources for the Machine Tool Industry. Course Syllabi, Instructor's Handbook, [and] Student Laboratory Manual. INSTITUTION Texas State Technical Coll. System, Waco. SPONS AGENCY Office of Vocational and Adult Education (ED), Washington, DC.; National Science Foundation, Arlington, VA. Div. of Undergraduate Education. PUB DATE 1998-00-00 NOTE 1508p.; For related documents, see ED 401 431-445 and CE 077 005-017. Product of the MASTER (Machine Tool Advanced Skills Technology Educational Resources) Consortium. CONTRACT DUE-9553716 AVAILABLE FROM Online at http://machinetool.tstc.edu PUB TYPE Guides - Classroom - Learner (051)-- Guides Classroom - Teacher (052) EDRS PRICE MF12/PC61 Plus Postage. DESCRIPTORS Behavioral Objectives; Competence; *Competency Based Education; Computer Assisted Design; *Computer Assisted Manufacturing; Consortia; Curriculum Design; Curriculum Guides; Employment Qualifications; Entry Workers; Equipment Utilization; Job Skills; Learning Activities; Learning Modules; *Machine Tool Operators; Machine Tools; Manufacturing Industry; Numerical Control; Postsecondary Education; *Production Technicians; *Technical Education; Vocational Education IDENTIFIERS DACUM Process; *Moldmaking ABSTRACT This package consists of a course syllabi, an instructor's handbook, and a student laboratory manual for a 2-year vocational training program to prepare students for entry-level employment as mold makers.The program was developed through a modification of the