Lysosomal Storage Disorders Diagnostic Algorithm, Part 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An in Vitro Model for Lysosomal Storage Diseases Using Aspartylglucosaminuria Patient Cells
AN IN VITRO MODEL FOR LYSOSOMAL STORAGE DISEASES USING ASPARTYLGLUCOSAMINURIA PATIENT CELLS Master´s Thesis University of Turku MSc Degree Programme in Biomedical Sciences Drug Discovery and Development 4/2020 Maria Kjellman Supervisors Riikka Äänismaa, PhD Senior Scientist Therapy Area Rare Diseases Orion Corporation Orion Pharma Jarkko Venäläinen, PhD Principal Scientist Therapy Area Rare Diseases Orion Corporation Orion Pharma Ullamari Pesonen, PhD Professor of Pharmacology and Drug Development Institute of Biomedicine Faculty of Medicine University of Turku Pharmacology, Drug Development and Therapeutics The originality of this thesis has been verified in accordance with the University of Turku quality assurance system using the Turnitin OriginalityCheck service. ABSTRACT UNIVERSITY OF TURKU Institute of Biomedicine, Faculty of Medicine KJELLMAN, MARIA: An In Vitro Model for Lysosomal Storage Diseases Using Aspartylglucosaminuria Patient Cells Master‘s Thesis, 73 pages MSc Degree Programme in Biomedical Sciences Drug Discovery and Development April 2020 BACKGROUND Lysosomes are acidic organelles responsible for recycling of metabolic byproducts and cellular debris, and there are approximately 60 enzymes within lysosomes responsible for the recycling process. These enzymes can be malfunctional due to genetic mutations, which results in a lysosomal storage disease (LSD). One of these diseases is aspartylglucosaminuria (AGU) caused by an incorrectly folded aspartylglucosaminidase (AGA) enzyme, which results in a buildup of the substrate aspartylglucosamine. This enzymatic deficiency impairs the cellular function in both central nervous system (CNS) and periphery manifesting as an impaired physical and mental development. UNMET MEDICAL NEED Currently, there are no curative or even symptom alleviating treatments available. AIM The aim of this research was to establish a reliable in vitro model of LSD to enable repeatable experiments for drug development purposes. -

Fast Urinary Screening of Oligosaccharidoses by MALDI-TOF/TOF Mass Spectrometry
Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry. Laurent Bonesso, Monique Piraud, Céline Caruba, Emmanuel van Obberghen, Raymond Mengual, Charlotte Hinault To cite this version: Laurent Bonesso, Monique Piraud, Céline Caruba, Emmanuel van Obberghen, Raymond Mengual, et al.. Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry.. Orphanet Journal of Rare Diseases, BioMed Central, 2014, 9 (1), pp.19. 10.1186/1750-1172-9-19. inserm- 00945684 HAL Id: inserm-00945684 https://www.hal.inserm.fr/inserm-00945684 Submitted on 12 Feb 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Bonesso et al. Orphanet Journal of Rare Diseases 2014, 9:19 http://www.ojrd.com/content/9/1/19 RESEARCH Open Access Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry Laurent Bonesso1, Monique Piraud5, Céline Caruba1, Emmanuel Van Obberghen1,2,3,4, Raymond Mengual1† and Charlotte Hinault1,2,3,4*† Abstract Background: Oligosaccharidoses, which belong to the lysosomal storage diseases, are inherited metabolic disorders due to the absence or the loss of function of one of the enzymes involved in the catabolic pathway of glycoproteins and indirectly of glycosphingolipids. -

4Th Glycoproteinoses International Conference Advances in Pathogenesis and Therapy
Program & Abstracts 4TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE ADVANCES IN PATHOGENESIS AND THERAPY ISMRD ST. LOUIS, MISSOURI, UNITED STATES Program & Abstracts I SM R D ADVANCES IN PATHOGENESIS AND THERAPY Program & Abstracts ISMRD would like to say A Very Special Thank You to the following organizations and companies who have very generously given donations and sponsorship to support the 4th International Conference on Glycoproteinoses THE PRENILLE EDWARD MALLINCKRODT FOUNDATION JR FOUNDATION MARK HASKINS I SM R D 4TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE 2015 ADVANCES IN PATHOGENESIS AND THERAPY Program & Abstracts ISMRD is very proud to display 10 featured Expression of Hope artworks to be Auctioned at the Gala Dinner. These beautiful prints are from Genzyme’s featured Artwork selection. Contents Welcome 1 SCIENTIFIC COMMITTEE: Stuart Kornfeld ISMRD Mission & Governance 3 (Chair, Scientifi c Planning Committee) Steve Walkley Sara Cathey ISMRD General Information 5 Richard Steet Sean Thomas Ackley, Philippines Miriam Storchli, Switzerland Alessandra d’Azzo ‘Hope’ by Sarah Noble, New Zealand Scientifi c Program 9 FAMILY CONFERENCE COMMITTEE: Family Program for Mucolipidosis 11 Jenny Noble (Conference Organiser) Jackie James (Conference Organiser Family Program For Alpha Mannosidosis /Sialidosis/ 13 - St. Louis) Fucosidosis/Aspartylglucosaminuria Mark Stark John Forman ‘All around the world’ by Zih Yun Li , Taiwan Childrens Program 16 Susan Kester Carolyn Paisley-Dew Tish Adkins Abstracts 17 Sara DeAngelis, Russia Gayle Rose, United States Speaker Profi les 60 Delegates 81 Helen Walker, Australia Nicklas Harkins, Canada Naomi Arai, Japan David Wentworth, Serbia I SM R D 4TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE 2015 ADVANCES IN PATHOGENESIS AND THERAPY Program & Abstracts On behalf of the Scientifi c Planning Committee, I want to extend a warm welcome to all the investigators and Welcome! families who have traveled to St. -

International Conference
5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE Rome, Italy November 1-4 2017 EMBRACING INNOVATION ADVANCING THE CURE PROGRAM & ABSTRACTS 5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE ROME, ITALY NOVEMBER 1-4 2017 EMBRACING INNOVATION ADVANCING THE CURE ISMRD would like to say a very special thank you to the following organizations and companies who have very generously given donations to support the 5th International Conference on Glycoproteinoses. ISMRD is an internationally focused not-for-profi t organization whose mission is to advocate for families and patients aff ected by one of the following disorders. Alpha-Mannosidosis THE WAGNER FOUNDATION Aspartylglucosaminuria Beta-Mannosidosis Fucosidosis Galactosialidosis ISMRD is very grateful for all the help and support that Symposia has given us Sialidosis (Mucolipidosis I) in the organization of our Conference on-the-ground support in Rome. Mucolipidosis II, II/III, III alpha/beta Mucolipidosis III Gamma Schindler Disease EMBRACING INNOVATION ADVANCING THE CURE SCIENTIFIC COMMITTEE: Alessandra d’Azzo CHAIR Contents Amelia Morrone Italy Richard Steet USA Welcome 2 Heather Flanagan-Steet USA ISMRD Mission & Governance 4 Dag Malm Norway ISMRD General Information 6 Thomas Braulke Dedicated to helping patients Germany in the rare disease community Stuart Kornfeld with unmet medical needs Scientifi c Program 10 USA Ultragenyx Pharmaceutical Inc. is a clinical-stage Family Program 14 ISMRD CONFERENCE biopharmaceutical company committed to creating new COMMITTEE: therapeutics to combat serious, -

Oligosaccharide Screen, Urine
Lab Dept: Urine/Stool Test Name: OLIGOSACCHARIDE SCREEN, URINE General Information Lab Order Codes: OLGO Synonyms: N/A CPT Codes: 84376 – Sugars (mono-, di-, oligosaccharides); single qualitative, each specimen Test Includes: This is a screening method for a subset of lysosomal storage disorders including: alpha-mannosidosis, aspartylglucosaminuria, fucosidosis, Schindler disease, GM1 gangliosidosis, Sandhoff disease, sialidosis, galactosialidosis, mucolipidoses types II and III, and Pompe disease. Logistics Test Indications: Investigation of possible oligosaccharidoses Lab Testing Sections: Chemistry - Sendouts Referred to: Mayo Medical Laboratories (MML Test: OLIGU) Phone Numbers: MIN Lab: 612-813-6280 STP Lab: 651-220-6550 Test Availability: Daily, 24 hours Turnaround Time: 4 – 8 days; performed Monday and Wednesday Special Instructions: Include family history, clinical condition (asymptomatic or acute episode), diet, and drug therapy information. Specimen Specimen Type: Urine, random collection Container: Plastic leakproof container (No preservatives) Draw Volume: 8 mL (Minimum: 2 mL) urine Processed Volume: Same as Draw Volume Collection: A random urine sample may be obtained by voiding into a urine cup and is often performed at the laboratory. Bring the refrigerated container to the lab. Make sure all specimens submitted to the laboratory are properly labeled with the patient’s name, medical record number and date of birth. Special Processing: Lab Staff: Aliquot specimen into a 13 mL urine tube, no preservative. Freeze immediately. Store and ship at frozen temperatures. Patient Preparation: None Sample Rejection: Mislabeled or unlabeled specimens Interpretive Reference Range: An interpretive report will be provided. This is a screening test; not all oligosachharidoses are detected. The resulting excretion profile may be characteristic of a specific disorder; however, abnormal results require confirmation by enzyme assay or molecular genetic testing. -

Allo HCT for Metabolic Disorders and Severe Osteopetrosis Status: Recruiting
MT2013-31: Allo HCT for Metabolic Disorders and Severe Osteopetrosis Status: Recruiting Eligibility Criteria Sex: All Age: up to 55 Years old This study is NOT accepting healthy volunteers Inclusion Criteria: • 0 through 55 years of age • Adequate graft available • Adequate organ function • Eligible Diseases: • Mucopolysaccharidosis Disorders: • MPS IH (Hurler syndrome) • MPS II (Hunter syndrome) if the patient has no or minimal evidence of symptomatic neurologic disease but is expected to have a neurologic phenotype • MPS VI (Maroteaux-Lamy syndrome) • MPS VII (Sly syndrome) • Glycoprotein Metabolic Disorders: • Alpha mannosidosis • Fucosidosis • Aspartylglucosaminuria • Sphingolipidoses and Recessive Leukodystrophies: • Globoid cell leukodystrophy • Metachromatic leukodystrophy • Niemann-Pick B patients (sphingomyelin deficiency) • Niemann-Pick C subtype 2 • Peroxisomal Disorders: • Adrenoleukodystrophy with cerebral involvement • Zellweger syndrome • Neonatal Adrenoleukodystrophy • Infantile Refsum disease • Acyl-CoA-Oxidase Deficiency • D-Bifunctional enzyme deficiency • Multifunctional enzyme deficiency • Alpha-methylacyl-CoA Racmase Deficiency (AMACRD) • Mitochondrial Neurogastrointestingal Encephalopathy (MNGIE) • Severe Osteopetrosis (OP) • Hereditary Leukoencephalopathy with axonal spheroids (HDLS; CSF1R mutation) • Other Inherited Metabolic Disorders (IMD): Patients will also be considered who have other life-threatening, rare lysosomal, peroxisomal or other similar inherited disorders characterized by white matter disease or -
Pdf NTSAD Chart of Allied Diseases
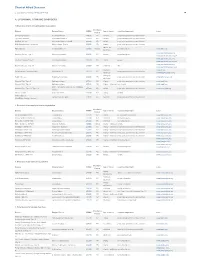
Chart of Allied Diseases Last Updated: Monday, 19 May 2014 17:02 A. LYSOSOMAL STORAGE DISORDERS 1) Disorders of lipid and sphingolipid degradation Inheritance Disease Enzyme Defect OMIM# Age of Onset Cognitive Impairment Links Pattern GM1 Gangliosidosis b-Galactosidase-1 230500 AR variable progressive psychomotor deterioration Tay-Sachs Disease b-Hexosaminidase A 272800 AR variable progressive psychomotor deterioration Sandhoff Disease b-Hexosaminidases A and B 268800 AR variable progressive psychomotor deterioration GM2 Gangliodisosis, AB variant GM2 Activator Protein 272750 AR infancy progressive psychomotor deterioration adolesence - Fabry Disease 8-Galactosidase A 301500 X-linked normal intelligence www.fabry.org adulthood www.gaucherdisease.org, Gaucher Disease, Type 1 Glucocerebrosidase 230800 AR variable normal intelligence www.gaucherdisease.org.uk www.gaucherdisease.org, Gaucher Disease, Type II Glucocerebrosidase 230900 AR infancy severe www.gaucherdisease.org.uk www.gaucherdisease.org, Gaucher Disease, Type III Glucocerebrosidase 231000 AR childhood mild www.gaucherdisease.org.uk infancy to www.ulf.org, Metachromatic Leukodystrophy Arylsulfatase A 250100 AR progressive psychomotor deterioration adulthood www.MLDFoundation.org infancy to Krabbe Disease Galactosylceramidase 245200 AR progressive psychomotor deterioration www.huntershope.org adulthood Niemann-Pick, Type A Sphingomyelinase 257200 AR infancy progressive psychomotor deterioration www.nnpdf.org Niemann-Pick, Type B Sphingomyelinase 607616 AR infancy - childhood none -

The Lysosomal Sialic Acid Transporter Sialin Is Required for Normal CNS Myelination
The Journal of Neuroscience, December 9, 2009 • 29(49):15355–15365 • 15355 Neurobiology of Disease The Lysosomal Sialic Acid Transporter Sialin Is Required for Normal CNS Myelination Laura M. Prolo,1 Hannes Vogel,2 and Richard J. Reimer1 1Department of Neurology and Neurological Sciences and Graduate Program in Neuroscience and 2Departments of Pathology and Pediatrics, Stanford University School of Medicine, Stanford, California 94305 Salla disease and infantile sialic acid storage disease are autosomal recessive lysosomal storage disorders caused by mutations in the gene encoding sialin, a membrane protein that transports free sialic acid out of the lysosome after it is cleaved from sialoglycoconjugates undergoing degradation. Accumulation of sialic acid in lysosomes defines these disorders, and the clinical phenotype is characterized by neurodevelopmental defects, including severe CNS hypomyelination. In this study, we used a sialin-deficient mouse to address how loss of sialin leads to the defect in myelination. Behavioral analysis of the sialin ؊/؊ mouse demonstrates poor coordination, seizures, and premature death. Analysis by histology, electron microscopy, and Western blotting reveals a decrease in myelination of the CNS but normal neuronal cytoarchitecture and normal myelination of the PNS. To investigate potential mechanisms underlying CNS hypomyeli- nation, we studied myelination and oligodendrocyte development in optic nerves. We found reduced numbers of myelinated axons in optic nerves from sialin ؊/؊ mice, but the myelin that was present appeared grossly normal. Migration and density of oligodendrocyte precursorcellswerenormal;however,amarkeddecreaseinthenumberofpostmitoticoligodendrocytesandanassociatedincreaseinthe number of apoptotic cells during the later stages of myelinogenesis were observed. These findings suggest that a defect in maturation of cells in the oligodendrocyte lineage leads to increased apoptosis and underlies the myelination defect associated with sialin loss. -

A Case Report
Open Access Case Report DOI: 10.7759/cureus.6139 Diagnosis and Supportive Management of Fucosidosis: A Case Report Arpanjeet Kaur 1 , Arshdeep S. Dhaliwal 2 , Hillary Raynes 3 , Thomas P. Naidich 4 , David M. Kaufman 3 1. General Medicine, Sibia Healthcare Pvt. Ltd., Sangrur, IND 2. General Medicine, Madhu Nursing Home, Patiala, IND 3. Pediatric Neurology, Mount Sinai Hospital, New York, USA 4. Radiology, Mount Sinai Hospital, New York, USA Corresponding author: Arshdeep S. Dhaliwal, [email protected] Abstract Fucosidosis is a lysosomal storage disease, resulting from a deficiency of the enzyme alpha-L-fucosidase. We present the case of an affected female with numerous manifestations, clinically and radiographically. In fucosidosis, advanced interventions are not always necessary to have rewarding outcomes. In fact, early diagnosis and management of the symptoms with a multi-systemic supportive care approach can improve the quality of life and may also prolong the life of those patients diagnosed with fucosidosis. Categories: Genetics, Neurology, Pediatrics Keywords: diagnosis, fucosidosis, fuca1, supportive management Introduction Fucosidosis is a very rare, autosomal, recessive genetic disorder associated with a mutation in the FUCA1 gene, resulting in a severe deficiency of the enzyme alpha-L-fucosidase in lysosomes. A total of 100 cases of fucosidosis have been reported until now [1-2]. Deficiency of this enzyme leads to the accumulation of fucose-containing glycolipids and other substances in various organs, which display a wide array of symptoms, such as severe global developmental delay (95%), muscle stiffness (87%), coarse facies (79%), recurrent respiratory infections (78%), abnormal bone development (58%), tortuous conjunctival vessels (53%), clusters of enlarged blood vessels on the skin (52%), abnormal enlargement of visceral organs (44%), and seizures (38%) [3-6]. -
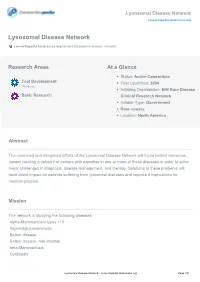
Lysosomal Disease Network Consortiapedia.Fastercures.Org
Lysosomal Disease Network consortiapedia.fastercures.org Lysosomal Disease Network consortiapedia.fastercures.org/consortia/lysosomal-disease-network/ Research Areas At a Glance Status: Active Consortium Tool Development Year Launched: 2004 Resource Initiating Organization: NIH Rare Disease Basic Research Clinical Research Network Initiator Type: Government Rare disease Location: North America Abstract The combined and integrated efforts of the Lysosomal Disease Network will focus limited resources toward creating a network of centers with expertise in one or more of these diseases in order to solve major challenges in diagnosis, disease management, and therapy. Solutions to these problems will have direct impact on patients suffering from lysosomal diseases and important implications for medical practice. Mission The network is studying the following diseases: alpha-Mannosidosis types I / II Aspartylglucosaminuria Batten disease Batten disease, late infantile beta-Mannosidosis Cystinosis Lysosomal Disease Network - consortiapedia.fastercures.org Page 1/5 Lysosomal Disease Network consortiapedia.fastercures.org Danon disease Fabry disease Farber disease Fucosidosis Galactosialidosis types I / II Gaucher disease GM1-Gangliosidosis types I/II/III GM2-Gangliosidosis Hunter syndrome Hurler syndrome I-cell disease Krabbe disease Maroteaux-Lamy syndrome Metachromatic leukodystrophy Morquio syndrome Mucolipidosis type IV Mucopolysaccharidosis type IX Multiple sulfatase deficiency Niemann-Pick disease Northern Epilepsy Pompe disease pseudo-Hurler -

Human Xc-N-Acetylgalactosaminidase Qx-NAGA)
4585 JMed Genet 1996;33:458-464 Human xc-N-acetylgalactosaminidase Qx-NAGA) deficiency: new mutations and the paradox J Med Genet: first published as 10.1136/jmg.33.6.458 on 1 June 1996. Downloaded from between genotype and phenotype J L M Keulemans, A J J Reuser, M A Kroos, R Willemsen, M M P Hermans, A M W van den Ouweland, J G N de Jong, R A Wevers, W 0 Renier, D Schindler, M J Coll, A Chabas, H Sakuraba, Y Suzuki, 0 P van Diggelen Abstract reported the second independent case of a- Up to now eight patients with a-NAGA NAGA deficiency with an entirely different deficiency have been described. This in- clinical phenotype. This patient had a late onset cludes the newly identified patient re- disease with slight facial coarseness, dis- ported here who died unexpectedly aged seminated angiokeratoma, and mild intellectual II years of hypoxia during convulsions; impairment (IQ= 70), but without neuro- necropsy was not performed. logical symptoms. Unlike the infantile cases, Three patients have been genotyped pre- this patient had prominent vacuolisation in all viously and here we report the mutations dermal cells, most prominently in vascular and in the other five patients, including two lymphatic endothelial cells and eccrine sweat new mutations (S160C and E193X). The gland cells, but also in dermal neural cells and newly identified patient is consanguineous fibroblasts.' The glomerular endothelial cells with the first patients reported with a- but not the epithelial kidney cells are involved NAGA deficiency and neuroaxonal dys- and also blood lymphocytes are vacuolised.6 trophy and they all had the a-NAGA geno- These three patients shared, however, the type E325KIE325K. -

The Myriad Foresight® Carrier Screen
The Myriad Foresight® Carrier Screen 180 Kimball Way | South San Francisco, CA 94080 www.myriadwomenshealth.com | [email protected] | (888) 268-6795 The Myriad Foresight® Carrier Screen - Disease Reference Book 11-beta-hydroxylase-deficient Congenital Adrenal Hyperplasia ...............................................................................................................................................................................8 6-pyruvoyl-tetrahydropterin Synthase Deficiency....................................................................................................................................................................................................10 ABCC8-related Familial Hyperinsulinism..................................................................................................................................................................................................................12 Adenosine Deaminase Deficiency ............................................................................................................................................................................................................................14 Alpha Thalassemia ....................................................................................................................................................................................................................................................16 Alpha-mannosidosis ..................................................................................................................................................................................................................................................18