4Th Glycoproteinoses International Conference Advances in Pathogenesis and Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuroradiologic Findings in Fucosidosis, a Rare Lysosomal Storage Disease
Neuroradiologic Findings in Fucosidosis, a Rare Lysosomal Storage Disease James M. Provenzale, Daniel P. Barboriak, and Katherine Sims Summary: Fucosidosis is a rare lysosomal storage disorder with vacuolated secondary lysosomes containing some fine the clinical features of mental retardation, cardiomegaly, dysos- fibrillar material and lamellated membrane structures. tosis multiplex, progressive neurologic deterioration, and early A magnetic resonance (MR) examination at the age of death. The neuroradiologic findings in two patients are reported, 10 years showed confluent regions of hyperintense signal and include abnormalities within the globus pallidus (both pa- on T2-weighted images in the periventricular regions, tients) and periventricular white matter (one patient). most prominent surrounding the atria of the lateral ventri- cles. Hyperintense signal was noted in the globus pallidus Index terms: Brain, metabolism; Degenerative disease, Pediatric on T1-weighted images, with corresponding hypointense neuroradiology signal on T2-weighted images (Fig 1). Fucosidosis is a rare metabolic disorder caused by decreased amounts of the enzyme Case 2 a-L-fucosidase, which results in the accumula- A 2-year-old boy was examined for speech delay and tion of fucose-rich storage products within psychomotor retardation. He was born at term after a many organs, including the brain. Patients with normal pregnancy, and appropriate development oc- this disorder usually have delayed intellectual curred during the first year of life. However, by age 2 years, and motor development, and often die within the patient had not developed speech, exhibited autistic the first decade of life. Computed tomographic behavior, and performed motor tasks poorly. Physical ex- (CT) findings have been reported in a few cases amination findings included coarsened facial features, nar- (1, 2). -

Program Download
5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE ROME, ITALY NOVEMBER 1-4 2017 EMBRACING INNOVATION ADVANCING THE CURE ISMRD would like to say a very special thank you to the following organizations and companies who have very generously given donations to support the 5th International Conference on Glycoproteinoses. THE WAGNER FOUNDATION ISMRD is very grateful for all the help and support that Symposia has given us in the organization of our Conference on-the-ground support in Rome. Dedicated to helping patients in the rare disease community with unmet medical needs Ultragenyx Pharmaceutical Inc. is a clinical-stage biopharmaceutical company committed to creating new therapeutics to combat serious, debilitating diseases. www.ultragenyx.com © 2017 Ultragenyx Pharmaceutical Inc. www.ultragenyx.com 8/16 MRCP-UGNX-00008 5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE 2017 Welcome to Rome! On behalf of ISMRD, I would like to welcome Of course, our conference would not take you all to the Fifth Glycoproteinoses place without significant charitable donations. International Conference on 'Embracing I would like to thank the following companies Innovation and Advancing the Cure'. and foundations, Ultragenyx, EveryLife Foundation, Sanofi/Genzyme, Amicus and The ISMRD is thrilled to bring our International Wagner Foundation. I would also like to extend Conference to Europe allowing us to connect our very grateful thanks to Symposia who are with families, researchers, clinicians, support event planners, who have worked alongside us groups and others who work in the field of rare here in Rome and have helped you check in at diseases. But, more importantly to help make registration. They will be on site throughout the the invaluable connections for families who meeting offering support and answering any perhaps have never met another family with questions you may have. -

File Download
Radiological and clinical characterization of the lysosomal storage disorders: non-lipid disorders Minzhi Xing, Emory University E I Parker, Emory University A Moreno-De-Luca, Geisinger Health System Elie Harmouche, Emory University Michael Terk, Emory University Journal Title: British Journal of Radiology Volume: Volume 87, Number 1033 Publisher: British Institute of Radiology | 2014-01-01, Pages 20130467-20130467 Type of Work: Article | Final Publisher PDF Publisher DOI: 10.1259/bjr.20130467 Permanent URL: https://pid.emory.edu/ark:/25593/pr2p5 Final published version: http://dx.doi.org/10.1259/bjr.20130467 Copyright information: © 2014 The Authors. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/), which permits distribution of derivative works, making multiple copies, distribution, public display, and publicly performance, provided the original work is properly cited. This license requires credit be given to copyright holder and/or author, copyright and license notices be kept intact. Accessed September 24, 2021 3:09 PM EDT BJR © 2014 The Authors. Published by the British Institute of Radiology Received: Revised: Accepted: doi: 10.1259/bjr.20130467 28 July 2013 29 October 2013 8 November 2013 Cite this article as: Xing M, Parker EI, Moreno-De-Luca A, Harmouche E, Terk MR. Radiological and clinical characterization of the lysosomal storage disorders: non-lipid disorders. Br J Radiol 2014;87:20130467. REVIEW ARTICLE Radiological -

Childhood Dementia Initiative
Childhood Dementia in Australia: quantifying the burden on patients, carers, the healthcare system and our society Prepared by: Dominic Tilden, Madeline Valeri and Magda Ellis THEMA Consulting Pty Ltd www.thema.net Prepared for: Childhood Dementia Initiative www.childhooddementia.org THEMA Consulting Report (2020). Childhood Dementia in Australia: quantifying the burden on patients, carers, the healthcare system and our society. www.childhooddementia.org/burdenstudy Released on the 18th November 2020 Sydney, Australia. © Childhood Dementia Initiative 2020 Website address for further details. www.childhooddementia.org KEY POINTS : Childhood dementia is a recognised, albeit little known group of disorders, comprised of more than 70 individual genetic conditions. : Childhooddementiadisordersareprogressiveanddevastatinginnatureandposeasignificantlycomplex medicalchallenge,withpatientstypicallyrelyingonfulltimesupportivecareandextensivehealthcare services in the mid-later stages of the disease. : ThisstudydemonstratesforthefirsttimetheburdenassociatedwithchildhooddementiainAustralia, the tremendous negative impact it has on affected children, families and the community, and the resulting economic costs. : Effective therapeutic intervention remains an unmet clinical need for the vast majority of childhood dementia disorders. : It is estimated that the collective incidence of disorders that cause childhood dementia is 36 per 100,000 live births. This equates to 129 births in Australia each year. : In 2021, it is estimated there will be 2273 Australians -

Fast Urinary Screening of Oligosaccharidoses by MALDI-TOF/TOF Mass Spectrometry
Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry. Laurent Bonesso, Monique Piraud, Céline Caruba, Emmanuel van Obberghen, Raymond Mengual, Charlotte Hinault To cite this version: Laurent Bonesso, Monique Piraud, Céline Caruba, Emmanuel van Obberghen, Raymond Mengual, et al.. Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry.. Orphanet Journal of Rare Diseases, BioMed Central, 2014, 9 (1), pp.19. 10.1186/1750-1172-9-19. inserm- 00945684 HAL Id: inserm-00945684 https://www.hal.inserm.fr/inserm-00945684 Submitted on 12 Feb 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Bonesso et al. Orphanet Journal of Rare Diseases 2014, 9:19 http://www.ojrd.com/content/9/1/19 RESEARCH Open Access Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry Laurent Bonesso1, Monique Piraud5, Céline Caruba1, Emmanuel Van Obberghen1,2,3,4, Raymond Mengual1† and Charlotte Hinault1,2,3,4*† Abstract Background: Oligosaccharidoses, which belong to the lysosomal storage diseases, are inherited metabolic disorders due to the absence or the loss of function of one of the enzymes involved in the catabolic pathway of glycoproteins and indirectly of glycosphingolipids. -

Geting Processes
Translational Science of Rare Diseases 4 (2019) 133–157 133 DOI 10.3233/TRD-190047 IOS Press Practical management of lysosomal storage disorders (LSDs) Pranoot Tanpaiboona,b,∗ aAdvanced Diagnostics Genetics, Genomics and R&D, Quest Diagnostics, San Juan Capistrano, CA, USA bChildren’s National Rare Disease Institute, Michigan Ave NW, Washington, DC, USA Abstract. Lysosomal Storage Disorders (LSDs) comprise a group of disorders causing defects at the organelle and sub- organelle level with a wide range of pathophysiologies and clinical consequences. Signs and symptoms of LSDs involve multiple organ systems. The main pathological mechanism of most LSDs was previously thought to be cytotoxic effects of a specific storage substance secondary to functional impairment or insufficient lysosomal enzymes. Other pathophysiologic mechanisms of LSDs have been discovered such as dysfunction of cell signaling, disturbance of cell homeostasis, inflamma- tory process and dysfunction of autophagy. The goal of treatment is to balance equilibrium of the enzyme and the accumulated substance. Replacing deficient enzyme through exogenous enzyme replacement therapy (ERT) or stem cell transplantation has been the main method of treatment for several years. The ability of ERT to alleviate neurologic symptoms is limited owing to the inability of the exogenous enzyme to cross the blood-brain barrier. The benefit of stem cell transplantation on neurologic symptoms has been demonstrated for Hurler syndrome, but it is not clear for most LSDs. Other strategies, such as gene therapy, have been under development to overcome this limitation and provide a better outcome. Early treatment or pre-symptomatic treatment could also slow disease progression and improve prognosis. -

Lysosomal Diseases
THE GLYCOPROTEINOSES: SECOND INTERNATIONAL WORKSHOP ON ADVANCES IN PATHOGENESIS AND THERAPY JULY 26-27, 2007 ANN ARBOR, MICHIGAN Scientific Conference Day 1 Morning Sessions, July 26 7:30-8:15 Continental Breakfast 8:15-8:30 Welcome and Introductions, Opening Remarks – Goals of the conference, progress since the last conference - Steven Walkley (with additional comments by officials from ISMRD, NINDS, ORD) 8:30-9:15 Plenary Address Cross-Correction: Looking Back, Looking Forward – Elizabeth Neufeld Session 1: Animal Models, Pathogenesis, Experimental Therapies (Chair, Mark Haskins) α-Mannosidosis 9:15-9:35 Enzyme replacement therapy for α-Mannosidosis - Judith Blanz 9:35-9:55 α-Mannosidosis in the Guinea Pig – Pathophysiological, behavioral, and treatment issues - John Hopwood 10:00-10:15 Break α-Mannosidosis (continued) 10:15-10:35 Strategies for translating gene therapy in the brain from rodents to clinical use - John Wolfe 10:35-10:55 Quantitative Imaging of gray matter disease associated with feline α- mannosidosis – Charles Vite β-Mannosidosis 10:55-11:15 β-Mannosidosis mice – A model for the human disease? – Karen Friderici Fucosidosis 11:15-11:30 Intrathecal enzyme infusion therapy in canine fucosidosis – Gauthami Kondagari 11:30-12:00 General Discussion 12:00-1:30 Lunch Afternoon Session, July 26 1:30-2:15 Special Lecture: The Glycoproteinoses: Limited Progress in Molecular Insight still Surpasses Present-day Treatment Efficacy – Jules Leroy Session 2: Animal Models, Pathogenesis, Experimental Therapies (Chair, John Wolfe) -

Mucopolysaccharidoses and Mucolipidoses
J Clin Pathol: first published as 10.1136/jcp.s3-8.1.64 on 1 January 1974. Downloaded from J. clin. Path., 27, Suppl. (Roy. Coll. Path.), 8, 64-93 Mucopolysaccharidoses and mucolipidoses F. VAN HOOF From the Laboratoire de Chimie Physiologique, Universite Catholique de Louvain, and International Institute of Cellular and Molecular Pathology, Brussels, Belgium Many different syndromes are classified as muco- THE CHEMICAL ERA polysaccharidoses, and, despite remarkable progress Chemical studies, performed mainly by groups in the biochemical understanding of these diseases, working with A. Dorfman, in Chicago and K. much remains to be learned and many cases still Meyer, in New York, have provided most of the escape classification. new knowledge in the field by analysis of tissue and Mucopolysaccharidoses are inborn storage dis- urinary mucopolysaccharides in patients (Dorfman eases, characterized by a complex accumulation of and Lorincz, 1957; Meyer, Grumbach, Linker, and mucopolysaccharides and of glycolipids within the Hoffman, 1958; Meyer, Hoffman, Linker, lysosomes. Sixteen human diseases correspond to Grumbach, and Sampson, 1959). These provided the this definition, of which nine have been presently basis for the subdivision of the 'Hurler syndrome' explained by the deficiency of an acid hydrolase. into six subgroups (McKusick, Kaplan, Wise, They are somewhat arbitrarily divided into muco- Hanley, Suddarth, Sevick, and Maumanee, 1965). polysaccharidoses and mucolipidoses. In muco- The possibility that mucopolysaccharidoses could polysaccharidoses, mucopolysaccharides are the result from an excessive biosynthesis of muco- main storage substances, although an abnormal polysaccharides was suggested by Matalon and accumulation of complex lipids is practically always Dorfman (1966). copyright. disclosed at least by the ultiastructural examination. -

DIAGNOSIS and THERAPIES for MUCOPOLYSACCHARIDOSES by Francyne Kubaski a Dissertation Submitted to the Faculty of the University
DIAGNOSIS AND THERAPIES FOR MUCOPOLYSACCHARIDOSES by Francyne Kubaski A dissertation submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biological Sciences Spring 2017 © 2017 Francyne Kubaski All Rights Reserved DIAGNOSIS AND THERAPIES FOR MUCOPOLYSACCHARIDOSES by Francyne Kubaski Approved: __________________________________________________________ Robin W. Morgan, Ph.D. Chair of the Department of Biological Sciences Approved: __________________________________________________________ George H. Watson, Ph.D. Dean of the College of Arts and Sciences Approved: __________________________________________________________ Ann L. Ardis, Ph.D. Senior Vice Provost for Graduate and Professional Education I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: __________________________________________________________ Erica M. Selva, Ph.D. Professor in charge of dissertation I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: __________________________________________________________ Shunji Tomatsu, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: __________________________________________________________ Robert W. Mason, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. -

International Conference
5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE Rome, Italy November 1-4 2017 EMBRACING INNOVATION ADVANCING THE CURE PROGRAM & ABSTRACTS 5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE ROME, ITALY NOVEMBER 1-4 2017 EMBRACING INNOVATION ADVANCING THE CURE ISMRD would like to say a very special thank you to the following organizations and companies who have very generously given donations to support the 5th International Conference on Glycoproteinoses. ISMRD is an internationally focused not-for-profi t organization whose mission is to advocate for families and patients aff ected by one of the following disorders. Alpha-Mannosidosis THE WAGNER FOUNDATION Aspartylglucosaminuria Beta-Mannosidosis Fucosidosis Galactosialidosis ISMRD is very grateful for all the help and support that Symposia has given us Sialidosis (Mucolipidosis I) in the organization of our Conference on-the-ground support in Rome. Mucolipidosis II, II/III, III alpha/beta Mucolipidosis III Gamma Schindler Disease EMBRACING INNOVATION ADVANCING THE CURE SCIENTIFIC COMMITTEE: Alessandra d’Azzo CHAIR Contents Amelia Morrone Italy Richard Steet USA Welcome 2 Heather Flanagan-Steet USA ISMRD Mission & Governance 4 Dag Malm Norway ISMRD General Information 6 Thomas Braulke Dedicated to helping patients Germany in the rare disease community Stuart Kornfeld with unmet medical needs Scientifi c Program 10 USA Ultragenyx Pharmaceutical Inc. is a clinical-stage Family Program 14 ISMRD CONFERENCE biopharmaceutical company committed to creating new COMMITTEE: therapeutics to combat serious, -
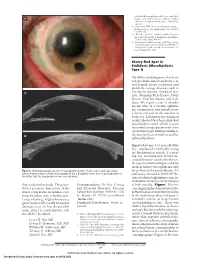
Mucolipidosis Type I)
endothelial keratoplasty in 200 eyes: early chal- A lenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32(3): 411-418. 4. Culbertson WW. Descemet stripping endothe- lial keratoplasy. Int Ophthalmol Clin. 2006;46 (3):155-168. 5. Ellis DR, Cohen KL. Sulfur hexafluoride gas in the repair of Descemet’s membrane detachment. Cornea. 1995;14(4):436-437. 6. Sorenson A. DSEK prolene pull. http://www .newmediamedicine.com/videos/2007/01/17 /dsek-prolene-pull-andrew-sorenson-md/. Ac- cessed March 18, 2007. Cherry Red Spot in Sialidosis (Mucolipidosis Type I) The differential diagnosis of a cherry red spot in the macula includes cen- tral retinal artery occlusion and metabolic storage diseases such as B Tay-Sachs disease, Sandhoff dis- ease, Niemann-Pick disease, Fabry disease, Gaucher disease, and siali- dosis. We report a case of an ado- lescent who, at a routine ophthal- mic examination, was found to have a cherry red spot in the maculae of both eyes. Laboratory investigation results showed that the patient had mucolipidosis type I, which is a rare lysosomal storage disease with clini- cal and histologic findings similar to C the mucopolysaccharidoses and the sphingolipidoses. Report of a Case. A 14-year-old white boy complained of difficulty seeing the blackboard at school. A screen- ing eye examination found de- creased distance vision in both eyes. He was of normal intelligence and his medical history was significant only Figure 3. Slitlamp photograph of case 3 at postoperative month 1 shows a clear cornea (A), and on for scoliosis and seasonal allergies. -

Alpha-Fucosidosis ¬タモ Two Brothers Presenting with Dysostosis Multiplex
The Egyptian Journal of Medical Human Genetics (2016) 17, 243–246 HOSTED BY Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com CASE REPORT Alpha-fucosidosis – Two brothers presenting with dysostosis multiplex Rimshah Shaukat a, Syed Musa Raza a, Zabedah Md. Yuns b, Affandi Omar b, Bushra Afroze c,* a Aga Khan Medical University, Karachi, Pakistan b Institute of Medical Research, Kuala Lumpur, Malaysia c Department of Paediatrics & Child Health, Aga Khan University Hospital, Karachi, Pakistan Received 5 November 2015; accepted 30 November 2015 Available online 30 December 2015 KEYWORDS Abstract a-Fucosidosis is a rare inherited neuro-degenerative disorder causing progressive neuro- a Alpha-fucosidosis; logical deterioration leading to early death. Definitive diagnosis requires -fucosidase enzyme assay Pakistani patients; or FUCA1 gene testing, which being expensive limits the definitive diagnosis in resource limited Dysostosis multiplex countries. We present two siblings with classic symptoms, radiological and MRI brain findings sug- gestive of a-fucosidosis and a clinical approach to reach to the diagnosis. Ó 2015 The Authors. Production and hosting by Elsevier B.V. on behalf of Ain Shams University. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). 1. Introduction a-fucosidosis from Pakistan, focusing on radiological findings as a diagnostic tool to develop an approach to a patient with a-Fucosidosis (OMIM # 230000) is an autosomal recessive dysostosis multiplex, in neuro-degenerative disorders including a lysosomal storage disorder caused by the deficiency of fucosi- -fucosidosis (Fig. 1).