Conventional and Unconventional Therapeutic Strategies for Sialidosis Type I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuroradiologic Findings in Fucosidosis, a Rare Lysosomal Storage Disease
Neuroradiologic Findings in Fucosidosis, a Rare Lysosomal Storage Disease James M. Provenzale, Daniel P. Barboriak, and Katherine Sims Summary: Fucosidosis is a rare lysosomal storage disorder with vacuolated secondary lysosomes containing some fine the clinical features of mental retardation, cardiomegaly, dysos- fibrillar material and lamellated membrane structures. tosis multiplex, progressive neurologic deterioration, and early A magnetic resonance (MR) examination at the age of death. The neuroradiologic findings in two patients are reported, 10 years showed confluent regions of hyperintense signal and include abnormalities within the globus pallidus (both pa- on T2-weighted images in the periventricular regions, tients) and periventricular white matter (one patient). most prominent surrounding the atria of the lateral ventri- cles. Hyperintense signal was noted in the globus pallidus Index terms: Brain, metabolism; Degenerative disease, Pediatric on T1-weighted images, with corresponding hypointense neuroradiology signal on T2-weighted images (Fig 1). Fucosidosis is a rare metabolic disorder caused by decreased amounts of the enzyme Case 2 a-L-fucosidase, which results in the accumula- A 2-year-old boy was examined for speech delay and tion of fucose-rich storage products within psychomotor retardation. He was born at term after a many organs, including the brain. Patients with normal pregnancy, and appropriate development oc- this disorder usually have delayed intellectual curred during the first year of life. However, by age 2 years, and motor development, and often die within the patient had not developed speech, exhibited autistic the first decade of life. Computed tomographic behavior, and performed motor tasks poorly. Physical ex- (CT) findings have been reported in a few cases amination findings included coarsened facial features, nar- (1, 2). -

An in Vitro Model for Lysosomal Storage Diseases Using Aspartylglucosaminuria Patient Cells
AN IN VITRO MODEL FOR LYSOSOMAL STORAGE DISEASES USING ASPARTYLGLUCOSAMINURIA PATIENT CELLS Master´s Thesis University of Turku MSc Degree Programme in Biomedical Sciences Drug Discovery and Development 4/2020 Maria Kjellman Supervisors Riikka Äänismaa, PhD Senior Scientist Therapy Area Rare Diseases Orion Corporation Orion Pharma Jarkko Venäläinen, PhD Principal Scientist Therapy Area Rare Diseases Orion Corporation Orion Pharma Ullamari Pesonen, PhD Professor of Pharmacology and Drug Development Institute of Biomedicine Faculty of Medicine University of Turku Pharmacology, Drug Development and Therapeutics The originality of this thesis has been verified in accordance with the University of Turku quality assurance system using the Turnitin OriginalityCheck service. ABSTRACT UNIVERSITY OF TURKU Institute of Biomedicine, Faculty of Medicine KJELLMAN, MARIA: An In Vitro Model for Lysosomal Storage Diseases Using Aspartylglucosaminuria Patient Cells Master‘s Thesis, 73 pages MSc Degree Programme in Biomedical Sciences Drug Discovery and Development April 2020 BACKGROUND Lysosomes are acidic organelles responsible for recycling of metabolic byproducts and cellular debris, and there are approximately 60 enzymes within lysosomes responsible for the recycling process. These enzymes can be malfunctional due to genetic mutations, which results in a lysosomal storage disease (LSD). One of these diseases is aspartylglucosaminuria (AGU) caused by an incorrectly folded aspartylglucosaminidase (AGA) enzyme, which results in a buildup of the substrate aspartylglucosamine. This enzymatic deficiency impairs the cellular function in both central nervous system (CNS) and periphery manifesting as an impaired physical and mental development. UNMET MEDICAL NEED Currently, there are no curative or even symptom alleviating treatments available. AIM The aim of this research was to establish a reliable in vitro model of LSD to enable repeatable experiments for drug development purposes. -

Program Download
5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE ROME, ITALY NOVEMBER 1-4 2017 EMBRACING INNOVATION ADVANCING THE CURE ISMRD would like to say a very special thank you to the following organizations and companies who have very generously given donations to support the 5th International Conference on Glycoproteinoses. THE WAGNER FOUNDATION ISMRD is very grateful for all the help and support that Symposia has given us in the organization of our Conference on-the-ground support in Rome. Dedicated to helping patients in the rare disease community with unmet medical needs Ultragenyx Pharmaceutical Inc. is a clinical-stage biopharmaceutical company committed to creating new therapeutics to combat serious, debilitating diseases. www.ultragenyx.com © 2017 Ultragenyx Pharmaceutical Inc. www.ultragenyx.com 8/16 MRCP-UGNX-00008 5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE 2017 Welcome to Rome! On behalf of ISMRD, I would like to welcome Of course, our conference would not take you all to the Fifth Glycoproteinoses place without significant charitable donations. International Conference on 'Embracing I would like to thank the following companies Innovation and Advancing the Cure'. and foundations, Ultragenyx, EveryLife Foundation, Sanofi/Genzyme, Amicus and The ISMRD is thrilled to bring our International Wagner Foundation. I would also like to extend Conference to Europe allowing us to connect our very grateful thanks to Symposia who are with families, researchers, clinicians, support event planners, who have worked alongside us groups and others who work in the field of rare here in Rome and have helped you check in at diseases. But, more importantly to help make registration. They will be on site throughout the the invaluable connections for families who meeting offering support and answering any perhaps have never met another family with questions you may have. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Fast Urinary Screening of Oligosaccharidoses by MALDI-TOF/TOF Mass Spectrometry
Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry. Laurent Bonesso, Monique Piraud, Céline Caruba, Emmanuel van Obberghen, Raymond Mengual, Charlotte Hinault To cite this version: Laurent Bonesso, Monique Piraud, Céline Caruba, Emmanuel van Obberghen, Raymond Mengual, et al.. Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry.. Orphanet Journal of Rare Diseases, BioMed Central, 2014, 9 (1), pp.19. 10.1186/1750-1172-9-19. inserm- 00945684 HAL Id: inserm-00945684 https://www.hal.inserm.fr/inserm-00945684 Submitted on 12 Feb 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Bonesso et al. Orphanet Journal of Rare Diseases 2014, 9:19 http://www.ojrd.com/content/9/1/19 RESEARCH Open Access Fast urinary screening of oligosaccharidoses by MALDI-TOF/TOF mass spectrometry Laurent Bonesso1, Monique Piraud5, Céline Caruba1, Emmanuel Van Obberghen1,2,3,4, Raymond Mengual1† and Charlotte Hinault1,2,3,4*† Abstract Background: Oligosaccharidoses, which belong to the lysosomal storage diseases, are inherited metabolic disorders due to the absence or the loss of function of one of the enzymes involved in the catabolic pathway of glycoproteins and indirectly of glycosphingolipids. -

4Th Glycoproteinoses International Conference Advances in Pathogenesis and Therapy
Program & Abstracts 4TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE ADVANCES IN PATHOGENESIS AND THERAPY ISMRD ST. LOUIS, MISSOURI, UNITED STATES Program & Abstracts I SM R D ADVANCES IN PATHOGENESIS AND THERAPY Program & Abstracts ISMRD would like to say A Very Special Thank You to the following organizations and companies who have very generously given donations and sponsorship to support the 4th International Conference on Glycoproteinoses THE PRENILLE EDWARD MALLINCKRODT FOUNDATION JR FOUNDATION MARK HASKINS I SM R D 4TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE 2015 ADVANCES IN PATHOGENESIS AND THERAPY Program & Abstracts ISMRD is very proud to display 10 featured Expression of Hope artworks to be Auctioned at the Gala Dinner. These beautiful prints are from Genzyme’s featured Artwork selection. Contents Welcome 1 SCIENTIFIC COMMITTEE: Stuart Kornfeld ISMRD Mission & Governance 3 (Chair, Scientifi c Planning Committee) Steve Walkley Sara Cathey ISMRD General Information 5 Richard Steet Sean Thomas Ackley, Philippines Miriam Storchli, Switzerland Alessandra d’Azzo ‘Hope’ by Sarah Noble, New Zealand Scientifi c Program 9 FAMILY CONFERENCE COMMITTEE: Family Program for Mucolipidosis 11 Jenny Noble (Conference Organiser) Jackie James (Conference Organiser Family Program For Alpha Mannosidosis /Sialidosis/ 13 - St. Louis) Fucosidosis/Aspartylglucosaminuria Mark Stark John Forman ‘All around the world’ by Zih Yun Li , Taiwan Childrens Program 16 Susan Kester Carolyn Paisley-Dew Tish Adkins Abstracts 17 Sara DeAngelis, Russia Gayle Rose, United States Speaker Profi les 60 Delegates 81 Helen Walker, Australia Nicklas Harkins, Canada Naomi Arai, Japan David Wentworth, Serbia I SM R D 4TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE 2015 ADVANCES IN PATHOGENESIS AND THERAPY Program & Abstracts On behalf of the Scientifi c Planning Committee, I want to extend a warm welcome to all the investigators and Welcome! families who have traveled to St. -

Common Differentially Expressed Genes and Pathways Correlating Both Coronary Artery Disease and Atrial Fibrillation
EXCLI Journal 2021;20:126-141– ISSN 1611-2156 Received: December 08, 2020, accepted: January 11, 2021, published: January 18, 2021 Supplementary material to: Original article: COMMON DIFFERENTIALLY EXPRESSED GENES AND PATHWAYS CORRELATING BOTH CORONARY ARTERY DISEASE AND ATRIAL FIBRILLATION Youjing Zheng, Jia-Qiang He* Department of Biomedical Sciences and Pathobiology, College of Veterinary Medicine, Virginia Tech, Blacksburg, VA 24061, USA * Corresponding author: Jia-Qiang He, Department of Biomedical Sciences and Pathobiology, Virginia Tech, Phase II, Room 252B, Blacksburg, VA 24061, USA. Tel: 1-540-231-2032. E-mail: [email protected] https://orcid.org/0000-0002-4825-7046 Youjing Zheng https://orcid.org/0000-0002-0640-5960 Jia-Qiang He http://dx.doi.org/10.17179/excli2020-3262 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). Supplemental Table 1: Abbreviations used in the paper Abbreviation Full name ABCA5 ATP binding cassette subfamily A member 5 ABCB6 ATP binding cassette subfamily B member 6 (Langereis blood group) ABCB9 ATP binding cassette subfamily B member 9 ABCC10 ATP binding cassette subfamily C member 10 ABCC13 ATP binding cassette subfamily C member 13 (pseudogene) ABCC5 ATP binding cassette subfamily C member 5 ABCD3 ATP binding cassette subfamily D member 3 ABCE1 ATP binding cassette subfamily E member 1 ABCG1 ATP binding cassette subfamily G member 1 ABCG4 ATP binding cassette subfamily G member 4 ABHD18 Abhydrolase domain -

Keyword Index
Gene Therapy (1998) 5, 1717–1719 1998 Stockton Press All rights reserved 0969-7128/98 $12.00 http://www.stockton-press.co.uk/gt Keyword Index Volume 5 1998 15-deoxyspergualin 85 (BC) bcl-2 1156 complex stability 137 (BC) 293 cells adenoviral vectors 563 (BC) bicistronic vectors 1566 (BC) computerized tomography 896 5-azacytidine 1299 bidirectional promoter 76 connexin43 1114, 1221, 1372 5-fluorocytosine 507 biodegradable microspheres 740 cord blood 233 6-hydroxydopamine (6-OHDA) 1650 biolistics 692 corneal endothelium 1347 99mTc 798 BMP-7 1098 coronary infusion 1472 bone repair 1098 costimulation 223 AAV 50, 820 bradykinin 630 costimulatory 965 AAV helper genes 938 brain 712 (BC), 820 Cre-mediated recombination 1047 AAV vector production 938 brain tumors 1122 Cre/loxP 684 adeno-associated virus 277 (BC), 1434, breast tumor 240 cyclophosphamide 189 1604, 1642 bronchoscopy 166 cystic fibrosis 91, 131 (BC), 181, 218, 309, adeno-associated virus (AAV) 166 bystander effect 105, 113, 605, 1114, 1221, 345, 583, 1488 adenosine deaminase 320 1372, 1705 cytidine deaminase 1545 adenoviral gene transfer 85 cytochrome P450 1070 adenoviral vectors 309, 869, 1251 C2C12 1014 cytokine gene 1462 adenovirus 8, 19, 131 (BC), 174, 339, 345, CAG 240 cytokine gene transfer 481 352, 473, 507, 605, 621, 630, 740, 747, cancer 728 (R), 1105, 1400, 1462 cytokine receptors 253 778, 938, 975, 995, 1023, 1038, 1130, cancer gene therapy 189, 451, 1171 cytokines 253, 869, 1400 1156, 1213, 1259, 1314, 1363, 1400, 1552, cancer vaccine 247, 1677 cytosine arabinoside 1545 -

International Conference
5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE Rome, Italy November 1-4 2017 EMBRACING INNOVATION ADVANCING THE CURE PROGRAM & ABSTRACTS 5TH GLYCOPROTEINOSES INTERNATIONAL CONFERENCE ROME, ITALY NOVEMBER 1-4 2017 EMBRACING INNOVATION ADVANCING THE CURE ISMRD would like to say a very special thank you to the following organizations and companies who have very generously given donations to support the 5th International Conference on Glycoproteinoses. ISMRD is an internationally focused not-for-profi t organization whose mission is to advocate for families and patients aff ected by one of the following disorders. Alpha-Mannosidosis THE WAGNER FOUNDATION Aspartylglucosaminuria Beta-Mannosidosis Fucosidosis Galactosialidosis ISMRD is very grateful for all the help and support that Symposia has given us Sialidosis (Mucolipidosis I) in the organization of our Conference on-the-ground support in Rome. Mucolipidosis II, II/III, III alpha/beta Mucolipidosis III Gamma Schindler Disease EMBRACING INNOVATION ADVANCING THE CURE SCIENTIFIC COMMITTEE: Alessandra d’Azzo CHAIR Contents Amelia Morrone Italy Richard Steet USA Welcome 2 Heather Flanagan-Steet USA ISMRD Mission & Governance 4 Dag Malm Norway ISMRD General Information 6 Thomas Braulke Dedicated to helping patients Germany in the rare disease community Stuart Kornfeld with unmet medical needs Scientifi c Program 10 USA Ultragenyx Pharmaceutical Inc. is a clinical-stage Family Program 14 ISMRD CONFERENCE biopharmaceutical company committed to creating new COMMITTEE: therapeutics to combat serious, -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -
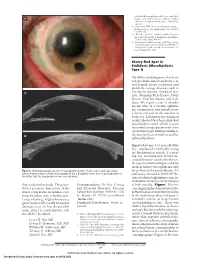
Mucolipidosis Type I)
endothelial keratoplasty in 200 eyes: early chal- A lenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32(3): 411-418. 4. Culbertson WW. Descemet stripping endothe- lial keratoplasy. Int Ophthalmol Clin. 2006;46 (3):155-168. 5. Ellis DR, Cohen KL. Sulfur hexafluoride gas in the repair of Descemet’s membrane detachment. Cornea. 1995;14(4):436-437. 6. Sorenson A. DSEK prolene pull. http://www .newmediamedicine.com/videos/2007/01/17 /dsek-prolene-pull-andrew-sorenson-md/. Ac- cessed March 18, 2007. Cherry Red Spot in Sialidosis (Mucolipidosis Type I) The differential diagnosis of a cherry red spot in the macula includes cen- tral retinal artery occlusion and metabolic storage diseases such as B Tay-Sachs disease, Sandhoff dis- ease, Niemann-Pick disease, Fabry disease, Gaucher disease, and siali- dosis. We report a case of an ado- lescent who, at a routine ophthal- mic examination, was found to have a cherry red spot in the maculae of both eyes. Laboratory investigation results showed that the patient had mucolipidosis type I, which is a rare lysosomal storage disease with clini- cal and histologic findings similar to C the mucopolysaccharidoses and the sphingolipidoses. Report of a Case. A 14-year-old white boy complained of difficulty seeing the blackboard at school. A screen- ing eye examination found de- creased distance vision in both eyes. He was of normal intelligence and his medical history was significant only Figure 3. Slitlamp photograph of case 3 at postoperative month 1 shows a clear cornea (A), and on for scoliosis and seasonal allergies. -

Oligosaccharide Screen, Urine
Lab Dept: Urine/Stool Test Name: OLIGOSACCHARIDE SCREEN, URINE General Information Lab Order Codes: OLGO Synonyms: N/A CPT Codes: 84376 – Sugars (mono-, di-, oligosaccharides); single qualitative, each specimen Test Includes: This is a screening method for a subset of lysosomal storage disorders including: alpha-mannosidosis, aspartylglucosaminuria, fucosidosis, Schindler disease, GM1 gangliosidosis, Sandhoff disease, sialidosis, galactosialidosis, mucolipidoses types II and III, and Pompe disease. Logistics Test Indications: Investigation of possible oligosaccharidoses Lab Testing Sections: Chemistry - Sendouts Referred to: Mayo Medical Laboratories (MML Test: OLIGU) Phone Numbers: MIN Lab: 612-813-6280 STP Lab: 651-220-6550 Test Availability: Daily, 24 hours Turnaround Time: 4 – 8 days; performed Monday and Wednesday Special Instructions: Include family history, clinical condition (asymptomatic or acute episode), diet, and drug therapy information. Specimen Specimen Type: Urine, random collection Container: Plastic leakproof container (No preservatives) Draw Volume: 8 mL (Minimum: 2 mL) urine Processed Volume: Same as Draw Volume Collection: A random urine sample may be obtained by voiding into a urine cup and is often performed at the laboratory. Bring the refrigerated container to the lab. Make sure all specimens submitted to the laboratory are properly labeled with the patient’s name, medical record number and date of birth. Special Processing: Lab Staff: Aliquot specimen into a 13 mL urine tube, no preservative. Freeze immediately. Store and ship at frozen temperatures. Patient Preparation: None Sample Rejection: Mislabeled or unlabeled specimens Interpretive Reference Range: An interpretive report will be provided. This is a screening test; not all oligosachharidoses are detected. The resulting excretion profile may be characteristic of a specific disorder; however, abnormal results require confirmation by enzyme assay or molecular genetic testing.