Microbiology Review: Bacteriological Cases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BD™ Gardnerella Selective Agar with 5% Human Blood
INSTRUCTIONS FOR USE – READY-TO-USE PLATED MEDIA PA-254094.06 Rev.: July 2014 BD Gardnerella Selective Agar with 5% Human Blood INTENDED USE BD Gardnerella Selective Agar with 5% Human Blood is a partially selective and differential medium for the isolation of Gardnerella vaginalis from clinical specimens. PRINCIPLES AND EXPLANATION OF THE PROCEDURE Microbiological method. Gardnerella vaginalis is considered to be one of the organisms causing vaginitis.1-4 Although the organism may be present in a high percentage of normal women in the vaginal flora, its importance as a cause of non-specific vaginitis (also called bacterial vaginosis) has never been questioned. In symptomatic women, G. vaginalis frequently is associated with anaerobes such as Prevotella bivia, P. disiens, Mobiluncus, Peptostreptococcus, and/or others which are a regular part of the urethral or intestinal, but not vaginal flora. In non-specific vaginitis, normal Lactobacillus flora is reduced or absent. Gardnerella vaginalis is considered to be the indicator organism for non-specific vaginitis which, in fact, is a polymicrobial infection.3,4 Although non- culture methods such as a direct Gram stain have been recommended in recent years for genital specimens, culture is still preferred by many laboratories.1,5 G. vaginalis may also be responsible for a variety of other diseases such as preterm birth, chorioamnionitis, urinary tract infections, newborn infections, and septicemia.6 The detection of the organism on routinely used media is difficult since Gardnerella and other -

CAMP Tests (Standard and Rapid) and Reverse CAMP Test
M.V.Sc. (Veterinary Microbiology), Monsoon semester Date 31.12.2020 VMC- 602 (Bacteriology II), Unit III, Practical class CAMP Tests (Standard and Rapid) and Reverse CAMP test Dr. Savita Kumari Assistant Professor-cum-Jr. Scientist Department of Veterinary Microbiology Bihar Veterinary College, BASU, Patna CAMP factor S. agalactiae contains the CAMP factor, only beta-hemolytic Streptococcus secretes Pore -forming toxin first identified in this bacterium CAMP reaction is based on the co -hemolytic activity of the CAMP factor Commonly used to identify S. agalactiae Closely related proteins present also in other Gram - positive pathogens cfb gene encodes CAMP factor CAMP test CAMP reaction- consists in a zone of strong hemolysis that is observed when S. agalactiae is streaked next to Staphylococcus aureus on blood agar S. aureus secretes sphingomyelinase Sheep red blood cells - rich in sphingomyelin, and upon exposure to sphingomyelinase become greatly sensitized to CAMP factor, which then effects hemolysis Hemolysis most pronounced in the zone between the colonies of the two bacterial species Co-hemolytic phenomenon- presumptive identification of Group B Streptococci (S. agalactiae) CAMP test First described by Christie, Atkins, and Munch –Petersen in 1944 The protein was named CAMP factor for the initials of the authors of the article that first described the phenomenon Types: Standard CAMP test Rapid CAMP test (spot test ) Standard camp test are time consuming and/or expensive compared to the CAMP spot test Principle CAMP test detects -

Use of the Diagnostic Bacteriology Laboratory: a Practical Review for the Clinician
148 Postgrad Med J 2001;77:148–156 REVIEWS Postgrad Med J: first published as 10.1136/pmj.77.905.148 on 1 March 2001. Downloaded from Use of the diagnostic bacteriology laboratory: a practical review for the clinician W J Steinbach, A K Shetty Lucile Salter Packard Children’s Hospital at EVective utilisation and understanding of the Stanford, Stanford Box 1: Gram stain technique University School of clinical bacteriology laboratory can greatly aid Medicine, 725 Welch in the diagnosis of infectious diseases. Al- (1) Air dry specimen and fix with Road, Palo Alto, though described more than a century ago, the methanol or heat. California, USA 94304, Gram stain remains the most frequently used (2) Add crystal violet stain. USA rapid diagnostic test, and in conjunction with W J Steinbach various biochemical tests is the cornerstone of (3) Rinse with water to wash unbound A K Shetty the clinical laboratory. First described by Dan- dye, add mordant (for example, iodine: 12 potassium iodide). Correspondence to: ish pathologist Christian Gram in 1884 and Dr Steinbach later slightly modified, the Gram stain easily (4) After waiting 30–60 seconds, rinse with [email protected] divides bacteria into two groups, Gram positive water. Submitted 27 March 2000 and Gram negative, on the basis of their cell (5) Add decolorising solvent (ethanol or Accepted 5 June 2000 wall and cell membrane permeability to acetone) to remove unbound dye. Growth on artificial medium Obligate intracellular (6) Counterstain with safranin. Chlamydia Legionella Gram positive bacteria stain blue Coxiella Ehrlichia Rickettsia (retained crystal violet). -

Human Microbiota Network: Unveiling Potential Crosstalk Between the Different Microbiota Ecosystems and Their Role in Health and Disease
nutrients Review Human Microbiota Network: Unveiling Potential Crosstalk between the Different Microbiota Ecosystems and Their Role in Health and Disease Jose E. Martínez †, Augusto Vargas † , Tania Pérez-Sánchez , Ignacio J. Encío , Miriam Cabello-Olmo * and Miguel Barajas * Biochemistry Area, Department of Health Science, Public University of Navarre, 31008 Pamplona, Spain; [email protected] (J.E.M.); [email protected] (A.V.); [email protected] (T.P.-S.); [email protected] (I.J.E.) * Correspondence: [email protected] (M.C.-O.); [email protected] (M.B.) † These authors contributed equally to this work. Abstract: The human body is host to a large number of microorganisms which conform the human microbiota, that is known to play an important role in health and disease. Although most of the microorganisms that coexist with us are located in the gut, microbial cells present in other locations (like skin, respiratory tract, genitourinary tract, and the vaginal zone in women) also play a significant role regulating host health. The fact that there are different kinds of microbiota in different body areas does not mean they are independent. It is plausible that connection exist, and different studies have shown that the microbiota present in different zones of the human body has the capability of communicating through secondary metabolites. In this sense, dysbiosis in one body compartment Citation: Martínez, J.E.; Vargas, A.; may negatively affect distal areas and contribute to the development of diseases. Accordingly, it Pérez-Sánchez, T.; Encío, I.J.; could be hypothesized that the whole set of microbial cells that inhabit the human body form a Cabello-Olmo, M.; Barajas, M. -
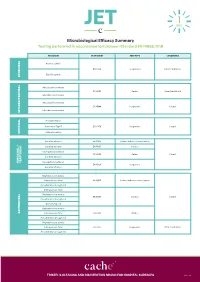
JET Microbiological Efficacy Summary
Microbiological Efficacy Summary Testing performed in accordance to European Standard EN 14885:2018 ORGANISM TEST NORM TEST TYPE CONDITIONS Bacillus subtilis EN 13704 Suspension Clean 1 and Dirty 1 Bacillus cereus SPORICIDAL Mycobacterium terrae EN 14563 Carrier Clean 1 and Dirty 2 Mycobacterium avium Mycobacterium terrae EN 14348 Suspension Clean 1 Mycobacterium avium MYCOBACTERICIDAL Poliovirus Type 1 Adenovirus Type 5 EN 14476 Suspension Clean 1 Murine Norovirus VIRUCIDAL Candida albicans EN 16615 Surface with mechanical action Candida albicans EN 13697 Surface Aspergillus brasiliensis EN 14562 Carrier Clean 1 Candida albicans YEASTICIDAL FUNGICIDAL / FUNGICIDAL Aspergillus brasiliensis EN 13624 Suspension Candida albicans Staphylococcus aureus Enterococcus hirae EN 16615 Surface with mechanical action Pseudomonas aeruginosa Enterococcus hirae Staphylococcus aureus EN 13697 Surface Clean 1 Pseudomonas aeruginosa Escherichia coli Staphylococcus aureus BACTERICIDAL Enterococcus hirae EN 14561 Carrier Pseudomonas aeruginosa Staphylococcus aureus Enterococcus hirae EN 13727 Suspension Clean 1 and Dirty 1 Pseudomonas aeruginosa TRISTEL’S CLEANING AND DISINFECTION BRAND FOR HOSPITAL SURFACES Page 1 of 3 Additional Testing TEST METHOD RNA DNA / Polyacrylamide gel electrophoresis (PAGE) ORGANISM TEST METHOD TEST TYPE CONDITIONS Acanthamoeba castellanii cysts Following the method of EN 13704 Suspension Clean 1 PROTOZOA Bacillus subtilis EN 17126 Suspension Clean 1 Bacillus cereus Clostridium difficile EN 13704 Suspension Clean 1 and Dirty 1 -

Newborn Colonization and Antibiotic Susceptibility Patterns of Streptococcus Agalactiae at the University of Gondar Referral
Gizachew et al. BMC Pediatrics (2018) 18:378 https://doi.org/10.1186/s12887-018-1350-1 RESEARCH ARTICLE Open Access Newborn colonization and antibiotic susceptibility patterns of Streptococcus agalactiae at the University of Gondar Referral Hospital, Northwest Ethiopia Mucheye Gizachew1*, Moges Tiruneh1, Feleke Moges1, Mulat Adefris2, Zemene Tigabu3 and Belay Tessema1 Abstract Background: Group B Streptococcus (GBS) that asymptomatically colonizing the recto-vaginal area of women is the most important cause of neonatal colonization. There is paucity of evidence about newborn colonization with GBS in Ethiopia. Thus, this study was aimed to determine the prevalence of newborn colonization with GBS, antibiotic susceptibility patterns of the isolates and associated risk factors at the University of Gondar Referral Hospital in Northwest Ethiopia Methods: A prospective cross sectional study was conducted from December 2016 to November 2017. A total of 1,155 swabs from nasal, ear and umbilical areas of the newborns were collected from the 385 newborns. Identifications of the isolates and antibiotic susceptibility testing were done by using conventional methods. Results: Sixty two (16.1%, 95% CI: 12.2% - 20%) of the newborns were colonized by GBS. Seven percent of the total specimens were positive for GBS. The antibiotics susceptibility rates of GBS (average of the three body sites tested) were 95.1%, 89.6%, 88.9%, 85.7%, 85.3%, 81.3%, 76.9%, 76.1%, 73.8%, and 34.4% to ampicillin, penicillin, ciprofloxacin, chloramphenicol, vancomycin, azitromycin, erythromycin, clindamycin, ceftriaxone, and tetracycline, respectively. A multilogistic regression analyses were shown that the newborns that were from mothers whose education status was below tertiary level, and newborns from mothers who were: being employed, being nullipara and multigravida were at risk for colonization with GBS. -

NOTES in Vitro Activities of Norfloxacin and Ciprofloxacin Against
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, July 1984, p. 94-96 Vol. 26, No. 1 0066-4804/84/070094-03$02.00/0 Copyright C 1984, American Society for Microbiology NOTES In Vitro Activities of Norfloxacin and Ciprofloxacin Against Mycobacterium tuberculosis, M. avium Complex, M. chelonei, M. fortuitum, and M. kansasii J. DOUGLAS GAY, DONALD R. DEYOUNG, AND GLENN D. ROBERTS* Section of Clinical Microbiology, Department of Laboratory Medicine, Mayo Clinic and Mayo Foundation, Rochester, Minnesota 55905 Received 28 November 1983/Accepted 4 April 1984 The activities of ciprofloxacin and norfloxacin against 100 mycobacteria isolates were studied in vitro by the 1% standard proportion method. Ciprofloxacin was more active against M. tuberculosis and M. fortuitum with MICs of 1.0 and 0.25 ,ug/ml, respectively, against 90% of isolates; norfloxacin had MICs of 8.0 and 2.0 ,ug/ml, respectively, against 90% of isolates. Nalidixic acid and other heterocyclic carbonic acid deriva- studied. The organisms were taken from the Mayo Clinic tives have been used primarily in the treatment of urinary stock culture collection, which included recent clinical iso- tract infections for many years. The compounds of this lates. Stock cultures were maintained on Middlebrook 7H10 general group include nalidixic acid, oxolinic acid, pipemidic agar slants (Difco Laboratories, Detroit, Mich.) and were acid, cinoxacin, and rosoxacin. Two new substances in this subcultured monthly. The identification of isolates was series which have been recently synthesized are norfloxacin based on standard biochemical tests (17) and gas-liquid (6) (1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-[ 1-piperazinyl ]-3- chromatography (16). -

TRYPTIC SOY AGAR - for in Vitro Use Only
TRYPTIC SOY AGAR - For in vitro use only - Plated Media Tubed Media PT80 –Tryptic Soy Agar (TSA) TT80 – TSA Slant PT81 – TSA (SXT) TT80-18 – TSA Pour Plate [18-mL] PT89 – TSA w Yeast Extract TB75 – TSA Blood Slant PB75 – TSA w 5% Sheep Blood PB81 – TSA w 7% Sheep Blood PB69 – TSA w 5% Horse Blood PB80 – TSA w 7% Horse Blood Tryptic Soy Agar (TSA) is a general purpose The CAMP test can also be used to help identify plating medium used for the isolation, cultivation, pathogenic species of Listeria . and maintenance of a variety of fastidious and non- TSA with horse blood is used to isolate more fastidious microorganisms. fastidious organisms. Horse blood contains both X Leavitt et al. demonstrated the versatility of and V factor, which are essential growth factors for TSA by cultivating both aerobic and anaerobic some organisms such as Haemophilus species. microbes using TSA. TSA is recognized and Sheep and human blood are not suitable since they recommended by numerous agencies around the contain specific enzymes that inactivate V Factor. world. Our standard formulation is prepared Although, some laboratories prefer a plated according to the United States Pharmacopeia medium with a higher blood content (7-10%) or (USP) and recommended for various different with horse blood, these mediums should not be applications put forth by the Association of used for determination of hemolytic reactions or Official Analytical Chemists (AOAC), the for the CAMP test. The increased blood content International Dairy Federation (IDF), the United can make hemolytic reactions less distinct and States Department of Agriculture (USDA), and the more difficult to read while defibrinated horse American Public Health Association (APHA). -

A Genomic Journey Through a Genus of Large DNA Viruses
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Virology Papers Virology, Nebraska Center for 2013 Towards defining the chloroviruses: a genomic journey through a genus of large DNA viruses Adrien Jeanniard Aix-Marseille Université David D. Dunigan University of Nebraska-Lincoln, [email protected] James Gurnon University of Nebraska-Lincoln, [email protected] Irina V. Agarkova University of Nebraska-Lincoln, [email protected] Ming Kang University of Nebraska-Lincoln, [email protected] See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/virologypub Part of the Biological Phenomena, Cell Phenomena, and Immunity Commons, Cell and Developmental Biology Commons, Genetics and Genomics Commons, Infectious Disease Commons, Medical Immunology Commons, Medical Pathology Commons, and the Virology Commons Jeanniard, Adrien; Dunigan, David D.; Gurnon, James; Agarkova, Irina V.; Kang, Ming; Vitek, Jason; Duncan, Garry; McClung, O William; Larsen, Megan; Claverie, Jean-Michel; Van Etten, James L.; and Blanc, Guillaume, "Towards defining the chloroviruses: a genomic journey through a genus of large DNA viruses" (2013). Virology Papers. 245. https://digitalcommons.unl.edu/virologypub/245 This Article is brought to you for free and open access by the Virology, Nebraska Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Virology Papers by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Adrien Jeanniard, David D. Dunigan, James Gurnon, Irina V. Agarkova, Ming Kang, Jason Vitek, Garry Duncan, O William McClung, Megan Larsen, Jean-Michel Claverie, James L. Van Etten, and Guillaume Blanc This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ virologypub/245 Jeanniard, Dunigan, Gurnon, Agarkova, Kang, Vitek, Duncan, McClung, Larsen, Claverie, Van Etten & Blanc in BMC Genomics (2013) 14. -

Streptococci
STREPTOCOCCI Streptococci are Gram-positive, nonmotile, nonsporeforming, catalase-negative cocci that occur in pairs or chains. Older cultures may lose their Gram-positive character. Most streptococci are facultative anaerobes, and some are obligate (strict) anaerobes. Most require enriched media (blood agar). Streptococci are subdivided into groups by antibodies that recognize surface antigens (Fig. 11). These groups may include one or more species. Serologic grouping is based on antigenic differences in cell wall carbohydrates (groups A to V), in cell wall pili-associated protein, and in the polysaccharide capsule in group B streptococci. Rebecca Lancefield developed the serologic classification scheme in 1933. β-hemolytic strains possess group-specific cell wall antigens, most of which are carbohydrates. These antigens can be detected by immunologic assays and have been useful for the rapid identification of some important streptococcal pathogens. The most important groupable streptococci are A, B and D. Among the groupable streptococci, infectious disease (particularly pharyngitis) is caused by group A. Group A streptococci have a hyaluronic acid capsule. Streptococcus pneumoniae (a major cause of human pneumonia) and Streptococcus mutans and other so-called viridans streptococci (among the causes of dental caries) do not possess group antigen. Streptococcus pneumoniae has a polysaccharide capsule that acts as a virulence factor for the organism; more than 90 different serotypes are known, and these types differ in virulence. Fig. 1 Streptococci - clasiffication. Group A streptococci causes: Strep throat - a sore, red throat, sometimes with white spots on the tonsils Scarlet fever - an illness that follows strep throat. It causes a red rash on the body. -

Sample Report Vaginosis.Pdf
LAB #: Sample Report CLIENT #: 12345 PATIENT: Sample Patient DOCTOR: Sample Doctor ID: Doctor's Data, Inc. SEX: Female 3755 Illinois Ave. DOB: 01/01/1993 AGE: 25 St. Charles, IL 60174 U.S.A. !"#$%&'(%(!)*'+%,- GRAM STAIN MICROSCOPY BACTERIAL VAGINOSIS SCORE Normal Abnormal Expected score interpretation: Lactobacilli None Mod - Many 0 - 3 BV not likely 4 - 6 BV indeterminate Curved Gram 10 7-10 BV highly suggestive Negative Rods Many None Small Gram 1 Many None The BV score is calculated based upon the gram stain results Negative Rods and is independent of the yeast, and bacterial cultures. 1Nugent Scoring System. (Nugent et al. J. Clin. Micro. Yeast None None (1991)29:297-301) RBC’s None None YEAST CULTURE 2+ Candida albicans WBC’s 0 0 - 6 Clue Cells Present None Eosinophils N/A None Eosinophils reported and Wrights Stain performed when WBC’s >6 Additional Gram Stain Findings: Rare Gram positive cocci in pairs BACTERIOLOGY CULTURE Expected/Beneficial flora Commensal (Imbalanced) flora Dysbiotic flora NG Lactobacillus spp. 2+ Alpha hemolytic strep 3+ Gardnerella vaginalis 2+ Gamma hemolytic strep 4+ Enterococcus faecalis 1+ Staphylococcus not aureus NG = No Growth SPECIMEN DATA Comments: Date Collected: 01/14/2019 Date Received: 01/16/2019 Date Completed: 01/23/2019 ©Doctor’s Data, Inc. !!! ADDRESS: 3755 Illinois Avenue, St. Charles, IL 60174-2420 !!! MED DIR: Erlo Roth, MD !!! CLIA ID NO: 14D0646470 0002038 LAB #: Sample Report CLIENT #: 12345 PATIENT: Sample DOCTOR: Sample Doctor Patient ID: Doctor's Data, Inc. SEX: Female 3755 Illinois Ave. DOB: 01/01/1993 St. Charles, IL 60174 U.S.A. -

Mycobacterium Avium Possesses Extracellular DNA That Contributes to Biofilm Formation, Structural Integrity, and Tolerance to Antibiotics
Mycobacterium avium Possesses Extracellular DNA that Contributes to Biofilm Formation, Structural Integrity, and Tolerance to Antibiotics Rose, S. J., Babrak, L. M., & Bermudez, L. E. (2015). Mycobacterium avium Possesses Extracellular DNA that Contributes to Biofilm Formation, Structural Integrity, and Tolerance to Antibiotics. PLoS ONE, 10(5), e0128772. doi: 10.1371/journal.pone.0128772 10.1371/journal.pone.0128772 Public Library of Science Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse RESEARCH ARTICLE Mycobacterium avium Possesses Extracellular DNA that Contributes to Biofilm Formation, Structural Integrity, and Tolerance to Antibiotics Sasha J. Rose1,2, Lmar M. Babrak1,2, Luiz E. Bermudez1,2* 1 Department of Biomedical Sciences, College of Veterinary Medicine, Oregon State University, Corvallis, Oregon, United States of America, 2 Department of Microbiology, College of Science, Oregon State University, Corvallis, Oregon, United States of America * [email protected] Abstract Mycobacterium avium subsp. hominissuis is an opportunistic pathogen that is associated with biofilm-related infections of the respiratory tract and is difficult to treat. In recent years, extracellular DNA (eDNA) has been found to be a major component of bacterial biofilms, in- OPEN ACCESS cluding many pathogens involved in biofilm-associated infections. To date, eDNA has not Citation: Rose SJ, Babrak LM, Bermudez LE (2015) been described as a component of mycobacterial biofilms. In this study, we identified and Mycobacterium avium Possesses Extracellular DNA characterized eDNA in a high biofilm-producing strain of Mycobacterium avium subsp. that Contributes to Biofilm Formation, Structural hominissuis (MAH). In addition, we surveyed for presence of eDNA in various MAH strains Integrity, and Tolerance to Antibiotics.