E-Binder March 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LGM-Pharma-Regulatory-1527671011
Pipeline Products List Specialty Portfolio Updated Q2 2018 Updated Q2 2018 See below list of newly approved API’s, samples are readily available for your R&D requirements: Inhalation Ophthalmic Transdermal Sublingual Abaloparatide Defibrotide Sodium Liraglutide Rituximab Abciximab Deforolimus Lixisenatide Rivastigmine Aclidinium Bromide Azelastine HCl Agomelatine Alprazolam Abemaciclib Delafloxacin Lumacaftor Rivastigmine Hydrogen Tartrate Beclomethasone Dipropionate Azithromycin Amlodipine Aripiprazole Acalabrutinib Denosumab Matuzumab Rizatriptan Benzoate Budesonide Besifloxacin HCl Apomorphine Eletriptan HBr Aclidinium Bromide Desmopressin Acetate Meloxicam Rocuronium Bromide Adalimumab Difluprednate Memantine Hydrochloride Rolapitant Flunisolide Bimatoprost Clonidine Epinephrine Aflibercept Dinoprost Tromethamine Micafungin Romidepsin Fluticasone Furoate Brimonidine Tartrate Dextromethorphan Ergotamine Tartrate Agomelatine Dolasetron Mesylate Mitomycin C Romosozumab Fluticasone Propionate Bromfenac Sodium Diclofenac Levocetrizine DiHCl Albiglutide Donepezil Hydrochloride Mometasone Furoate Rotigotine Formoterol Fumarate Cyclosporine Donepezil Meclizine Alectinib Dorzolamide Hydrochloride Montelukast Sodium Rucaparib Iloprost Dexamethasone Valerate Estradiol Melatonin Alemtuzumab Doxercalciferol Moxifloxacin Hydrochloride Sacubitril Alirocumab Doxorubicin Hydrochloride Mycophenolate Mofetil Salmeterol Xinafoate Indacaterol Maleate Difluprednate Fingolimod Meloxicam Amphotericin B Dulaglutide Naldemedine Secukinumab Levalbuterol Dorzolamide -

Pharmacokinetics, Pharmacodynamics and Drug
pharmaceutics Review Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies Danuta Szkutnik-Fiedler Department of Clinical Pharmacy and Biopharmacy, Pozna´nUniversity of Medical Sciences, Sw.´ Marii Magdaleny 14 St., 61-861 Pozna´n,Poland; [email protected] Received: 28 October 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug–drug (DDI) or drug–food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR). Keywords: migraine; lasmiditan; gepants; monoclonal antibodies; drug–drug interactions 1. Introduction Migraine is a chronic neurological disorder characterized by a repetitive, usually unilateral, pulsating headache with attacks typically lasting from 4 to 72 h. -

Commercial and Medicaid Formulary Changes Effective 1/1/21
Commercial and Medicaid formulary changes effective 1/1/21 These additions and changes apply to Commercial and Medicaid formularies and are effective 1/1/21 unless specified below. Additions Tecartus™ (brexucabtagene autoleucel) – Medical Benefit, PA Required (Reviewed by Medical Director). Rukobia®(fostemavir) – Non-Preferred Brand, QL Required. Phexxi (lactic acid, citric acid, and potassium bitartrate) – Non-Preferred Brand, QL Required. Viltepso (viltolarsen) – Medical Benefit, PA Required. Dayvigo (lemborexant) – Non-Preferred Brand, PA and QL Required. Barhemsys (amisulpride) – Medical Benefit. Blenrep (belanta mabmafodotin-blmf) – Medical Benefit, PA Required. Monjuvi (tafasitamab-cxix) – Medical Benefit, PA Required. Trijardy (empagliflozin/linagliptin/metformin) – Non-Preferred Brand, Step Therapy Required. Procysbiv (cysteamine) – Non-Preferred Brand, PA Required. Hemadyv (dexamethasone) – Non-Preferred Brand, PA and QL Required. Kynmobi (apomorphine) – Non-Preferred Brand, PA and QL Required. Zerviate (cetirizine) – Non-Preferred Brand, Step Therapy Required. Freestyle Libre 2 – Preferred Brand, PA and QL Required. Changes Belsomra (suvorexant) – Change to Non-Preferred Brand, PA and QL Required. Apokyn (apomorphine SC) – Change to Non-Preferred Brand, PA and QL Required. Copaxone (brand only) – Changed from Tier 3 to Tier 2 (only applies to commercial). Humalog (insulin lispro) – Changed from Tier 3 to Tier 2 (only applies to commercial). Nurtec ODT (rimegepant) – Changed from Tier 4 to Tier 3 (only applies to commercial). Reyvow (lasmiditan) – Change to Non-Preferred Brand, PA and QL Required (only applies to commercial). Ubrelvy (ubrogepant) – Change to Non-Preferred Brand, PA and QL Required (only applies to commercial). Soliqua (insulin glargine-lixisenatide) – Changed from Tier 4 to Tier 3 (only applies to commercial). Prefest (estradiol-norgestimate) – Moved to Non-formulary (only applies to commercial). -

Current and Prospective Pharmacological Targets in Relation to Antimigraine Action
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Erasmus University Digital Repository Naunyn-Schmiedeberg’s Arch Pharmacol (2008) 378:371–394 DOI 10.1007/s00210-008-0322-7 REVIEW Current and prospective pharmacological targets in relation to antimigraine action Suneet Mehrotra & Saurabh Gupta & Kayi Y. Chan & Carlos M. Villalón & David Centurión & Pramod R. Saxena & Antoinette MaassenVanDenBrink Received: 8 January 2008 /Accepted: 6 June 2008 /Published online: 15 July 2008 # The Author(s) 2008 Abstract Migraine is a recurrent incapacitating neuro- (CGRP1 and CGRP2), adenosine (A1,A2,andA3), glutamate vascular disorder characterized by unilateral and throbbing (NMDA, AMPA, kainate, and metabotropic), dopamine, headaches associated with photophobia, phonophobia, endothelin, and female hormone (estrogen and progesterone) nausea, and vomiting. Current specific drugs used in the receptors. In addition, we have considered some other acute treatment of migraine interact with vascular receptors, targets, including gamma-aminobutyric acid, angiotensin, a fact that has raised concerns about their cardiovascular bradykinin, histamine, and ionotropic receptors, in relation to safety. In the past, α-adrenoceptor agonists (ergotamine, antimigraine therapy. Finally, the cardiovascular safety of dihydroergotamine, isometheptene) were used. The last two current and prospective antimigraine therapies is touched decades have witnessed the advent of 5-HT1B/1D receptor upon. agonists (sumatriptan and second-generation triptans), which have a well-established efficacy in the acute Keywords 5-HT. Antimigraine drugs . CGRP. treatment of migraine. Moreover, current prophylactic Noradrenaline . Migraine . Receptors treatments of migraine include 5-HT2 receptor antagonists, Ca2+ channel blockers, and β-adrenoceptor antagonists. Despite the progress in migraine research and in view of its Introduction complex etiology, this disease still remains underdiagnosed, and available therapies are underused. -

Anti-Infectives Industry Over the Next 5 Years and Beyond
Bridging the innovation gap... New Drug Futures: Products that could change the pharma market to 2013 and beyond Over 70 pipeline prospects This new major and insightful 450 page in 8 major therapy areas analysis evaluates, compares and contrasts the are analysed in this report prospects for the development compounds that could revolutionise the pharmaceutical Anti-infectives industry over the next 5 years and beyond. Cardiovascular CNS The report provides: Gastrointestinal Detailed background and market context for Metabolic each therapy area covered: Musculoskeletal Addressable patient population Oncology Current treatments Sales drivers Respiratory Sales breakers Future treatments Market dynamics – winners and losers Key drug launches by 2013 Unique sales forecasts by major product to 2013 Over 70 key products assessed Unique evaluation scores for key areas such as novelty of mechanism, clinical data and competition Critical and detailed appraisal of each product‟s research and development Extensive pipeline listings, putting the profiled products into their competitive context The search – and need – for new products has never been greater and what’s in the development pipeline has never generated more interest. That is why this analysis is so important! GLOBAL PHARMA MARKET IN CONTEXT THE Are there too many prophets of doom ready to write-off the research-based pharma industry in the future? Too few novel There is plenty on which to base such anxiety. The research-based industry products and an must achieve a fair price in the face of greater cost control, while the aggressive generic burden of regulation is setting the bar high for successful product sector are taking introduction. -

NCT04179474 Study ID: 3110‐108‐002 Title: A
NCT04179474 Study ID: 3110‐108‐002 Title: A Phase 1b, Two‐Part, Open‐Label, Fixed‐Sequence, Safety, Tolerability and Drug‐Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Date: 14Aug2019 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Title Page Protocol Title: A Phase 1b, Two-Part, Open-Label, Fixed-Sequence, Safety, Tolerability and Drug-Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants with Migraine Protocol Number: 3110-108-002 Product: Ubrogepant (AGN-241688, MK-1602) Brief Protocol Title: Drug-Drug Interaction Study of Ubrogepant with Erenumab or Galcanezumab Study Phase: 1b Sponsor Name and Legal Registered Address: Allergan Pharmaceuticals International Limited, Clonshaugh Industrial Estate, Coolock, Dublin 17, Ireland US Agent: Allergan Sales, LLC, 2525 Dupont Dr, Irvine, California 92612, USA Regulatory Agency Identifying Number(s): IND #113924 SAE Reporting Fax Number/Email: Business Unit Email Phone Fax Allergan Approval Date: 14 August 2019 1 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Sponsor Signatories: Date Date The signatures of the sponsor signatories are collected on the protocol approval page. 2 GRD-CLN-T-01-01 v3.0 CONFIDENTIAL Protocol 3110-108-002 Ubrogepant (AGN-241688) Table of Contents Title Page .........................................................................................................................................1 -

Nurtec ODT (Rimegepant)
Market Applicability Market DC GA KY MD NJ NY WA Applicable X X X X X X X Nurtec ODT (rimegepant) Override(s) Approval Duration Prior Authorization 1 year Quantity Limit Medications Quantity Limit Nurtec ODT (rimegepant) 75 mg tablets 15 tablets per 30 days* *For approval of greater than 15 tablets per 30 days, the individual must meet the following criteria: I. Individual has a diagnosis of migraine headaches; AND II. Individual has had a previous trial (medication samples/coupons/discount cards are excluded from consideration as a trial) and an inadequate response to one of the following daily preventive therapies (AAN/AHA 2012/2015, ICSI 2013): A. A tricyclic antidepressant [such as but not limited to amitriptyline, doxepin]; OR B. A beta blocker [such as but not limited to metoprolol tartrate, propranolol, timolol, atenolol, nadolol, nebivolol]; OR C. A calcium channel blocker [such as but not limited to nicardipine, verapamil]; OR D. An ACE inhibitor [such as but not limited to lisinopril]; OR E. An angiotensin receptor blocker (ARBs) [such as but not limited to candesartan]; OR F. An alpha-2 agonist [such as but not limited to guanfacine]; OR G. An antiepileptic [such as but not limited to divalproex sodium, sodium valproate, topiramate, carbamazepine, gabapentin]; OR H. Other select antidepressants [such as but not limited to venlafaxine]; OR I. Cyproheptadine (Periactin). APPROVAL CRITERIA Requests for oral CGRP agents for acute migraine treatment (Nurtec ODT [rimegepant]) may be approved if the following criteria is met: I. Individual has had a trial (medication samples/coupons/discount cards are excluded from consideration as a trial) of and inadequate response or intolerance to two preferred oral triptans (AHS 2019); OR PAGE 1 of 2 08/07/2020 CRX-ALL-0575-20 New Program Date 03/19/2020 This policy does not apply to health plans or member categories that do not have pharmacy benefits, nor does it apply to Medicare. -

Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-Related Hypothalamic Neuropeptides
Georgia State University ScholarWorks @ Georgia State University Neuroscience Institute Dissertations Neuroscience Institute 8-7-2018 Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides Katherine MS West Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/neurosci_diss Recommended Citation West, Katherine MS, "Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides." Dissertation, Georgia State University, 2018. https://scholarworks.gsu.edu/neurosci_diss/36 This Dissertation is brought to you for free and open access by the Neuroscience Institute at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Neuroscience Institute Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. REGULATION OF VENTRAL TEGMENTAL AREA DOPAMINE NEURON ACTIVITY BY FEEDING-RELATED HYPOTHALAMIC NEUROPEPTIDES by KATHERINE M. S. WEST Under the Direction of Aaron Roseberry, Ph.D. ABSTRACT The prevalence of obesity has doubled worldwide since the 1980s, and having a high body mass index contributes to more deaths worldwide than being underweight. Over the past 20 years, consumption of calorie-dense foods has increased, and this is considered one of the major causes of the rapid rise in obesity. Thus, understanding the neural control of food intake is important for the development of new and effective treatments of obesity. Two important brain regions that regulate food intake are the hypothalamus and the mesocorticolimbic dopamine system. The hypothalamus is essential for the homeostatic control of feeding and body weight, while the mesocorticolimbic dopamine system, also known as the reward system, is the primary circuit for reward and motivated behavior. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -
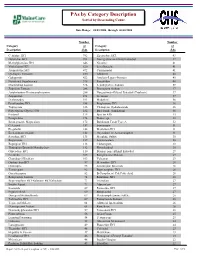
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

VYEPTI™ (EPTINEZUMAB) Policy Number: CSLA2020D0090A Effective Date: TBD
Proprietary Information of United Healthcare: The information contained in this document is proprietary and the sole property of United HealthCare Services, Inc. Unauthorized copying, use and distribution of this information are strictly prohibited. Copyright 2020 United HealthCare Services, Inc. UnitedHealthcare® Community Plan Medical Benefit Drug Policy VYEPTI™ (EPTINEZUMAB) Policy Number: CSLA2020D0090A Effective Date: TBD Table of Contents Page Commercial Policy APPLICATION ...................................................... 1 Vyepti™ (Eptinezumab) COVERAGE RATIONALE ........................................ 1 APPLICABLE CODES ............................................. 2 BACKGROUND ................................................... 43 CLINICAL EVIDENCE ............................................ 4 U.S. FOOD AND DRUG ADMINISTRATION ............ 54 CENTERS FOR MEDICARE AND MEDICAID SERVICES 54 REFERENCES ....................................................... 5 POLICY HISTORY/REVISION INFORMATION ...... 65 INSTRUCTIONS FOR USE .................................... 65 APPLICATION This Medical Benefit Drug Policy only applies to state of Louisiana. COVERAGE RATIONALE Vyepti has been added to the Review at Launch program. Please reference the Medical Benefit Drug Policy titled Review at Launch for New to Market Medications for additional details. Chronic Migraine Vyepti is proven and medically necessary for the preventive treatment of chronic migraines when all of the following criteria are met: For initial therapy, all of the -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading.