LGM-Pharma-Regulatory-1527671011
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2 Total Pharmaceutical Sales
OECD Health Statistics 2021 Definitions, Sources and Methods Total pharmaceutical sales Total sales of pharmaceutical products on the domestic market, in total and by selected Anatomical Therapeutic Chemical (ATC) classification groups, based on retail prices (which means the final price paid by the customer). The ATC codes below are based on the 2021 version of the ATC Index. All alterations implemented from January 2021 are available on the WHO Collaborating Centre for Drug Statistics Methodology website at http://www.whocc.no/atc/lists_of_new_atc_ddds_and_altera/alterations_in_atc_ddd/. Note: There are at least three possible sources of under-reporting of drug sales in different countries: 1) sales data may only cover those drugs that are reimbursed by public insurance schemes; 2) they may be based on ex-factory or wholesale prices rather than retail prices; and 3) sales data may exclude drug consumption in hospitals. Data for the following countries under-estimate pharmaceutical sales reported in this section because of one of these limitations: Australia, Austria, France, Germany, Greece, Japan, Luxembourg, the Netherlands, the Slovak Republic (before 2016) and Spain. (For further information, see the country-specific information below). Please also note that depending on the allocation of pharmaceutical products with more than one use, differences in reporting of specific drugs may occur across countries, thereby affecting the relative size of specific ATC groups. Data should reflect total sales for each drug category, based on -

Protocol Supplementary
Optimal Pharmacological Management and Prevention of Glucocorticoid-Induced Osteoporosis (GIOP) Protocol for a Systematic Review and Network Meta-Analysis Supplementary Materials: Sample Search Strategy Supplementary 1: MEDLINE Search Strategy Database: OVID Medline Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present Line 1 exp Osteoporosis/ 2 osteoporos?s.ti,ab,kf. 3 Bone Diseases, Metabolic/ 4 osteop?eni*.ti,ab,kf. 5 Bone Diseases/ 6 exp Bone Resorption/ 7 malabsorption.ti,ab,kf. 8 Bone Density/ 9 BMD.ti,ab,kf. 10 exp Fractures, Bone/ 11 fracture*.ti,ab,kf. 12 (bone* adj2 (loss* or disease* or resorption* or densit* or content* or fragil* or mass* or demineral* or decalcif* or calcif* or strength*)).ti,ab,kf. 13 osteomalacia.ti,ab,kf. 14 or/1-13 15 exp Glucocorticoids/ 16 exp Steroids/ 17 (glucocorticoid* or steroid* or prednisone or prednisolone or hydrocortisone or cortisone or triamcinolone or dexamethasone or betamethasone or methylprednisolone).ti,ab,kf. 18 or/15-17 19 14 and 18 20 ((glucocorticoid-induced or glucosteroid-induced or corticosteroid-induced or glucocorticosteroid-induced) adj1 osteoporos?s).ti,ab,kf. 21 19 or 20 22 exp Diphosphonates/ 23 (bisphosphon* or diphosphon*).ti,ab,kf. 24 exp organophosphates/ or organophosphonates/ 25 (organophosphate* or organophosphonate*).ti,ab,kf. 26 (alendronate or alendronic acid or Fosamax or Binosto or Denfos or Fosagen or Lendrate).ti,ab,kf. 27 (Densidron or Adrovance or Alenotop or Alned or Dronat or Durost or Fixopan or Forosa or Fosval or Huesobone or Ostemax or Oseolen or Arendal or Beenos or Berlex or Fosalen or Fosmin or Fostolin or Fosavance).ti,ab,kf. -

Maintenance Drug List
Commonly Prescribed Maintenance Medications Maintenance (or long-term) medications are those dosage form may be used to treat more than one drugs you may take on a regular basis to treat conditions medical condition. In these cases, each medication may such as high cholesterol, high blood pressure or diabetes. be classified according to its first U.S. Food and Drug Based on your benefit plan, you may get up to a 90-day Administration (FDA) approved use. Some strengths supply of covered maintenance medication delivered and/or formulations listed may not be covered. to you through a home delivery program or at select Please note that oral contraceptives are maintenance retail pharmacies. medications and are part of this list. However, this Some plans may require you to fill these prescriptions list does not include the trade names of all available through home delivery or at select retail pharmacies oral contraceptives because there are a large number in order to receive coverage. of products. Commonly used maintenance medications are listed in Coverage is always subject to the terms and limits of this guide. This list is not all-inclusive and may change your benefit plan. Please verify with your plan if there from time to time. are any additional requirements before a drug may be Most generic drugs listed are followed by a reference covered. For details about your plan, check your benefit brand drug in (parentheses). The brand name drug materials or call the number on your member ID card. in parentheses is listed for reference and may not be Third-party brand names are the property of their covered under your benefit. -

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Catalog HPD-5E ® CREATING A HEALTHY WORLDTM Active Pharmaceutical Ingredients (APIs) Available for International Markets Human Pharmaceutical Department www.Pharmapex.net Catalog HPD-5E *Not all products referred to on this site are available in all countries and our products are subject to different regulatory requirements depending on the country of use. Consequently, certain sections of this site may be indicated as being intended only for users in specic countries. Some of the products may also be marketed under different trade names. You should not construe anything on this site as a promotion or solicitation for any product or for the use of any product that is not authorized by the laws and regulations of your country of residence. For inquiries about the availability of any specic product in your country, you may simply contact us at [email protected]. **Products currently covered by valid US Patents may be offered for R&D use in accordance with 35 USC 271(e)+A13(1). Any patent infringement and resulting liability is solely at buyer risk. ©2016, Pharmapex USA, A member of Apex Group of Companies, All Rights Reserved. Toll-Free: 1.844.PHARMAPEX Fax: + 1.619.881.0035 ACTIVE PHARMACEUTICAL [email protected] CREATING A HEALTHY WORLD™ www.Pharmapex.net INGREDIENTS About Pharmapex’s Human Pharmaceuticals Department: Pharmapex’s Human Pharmaceuticals Department (HPD) is a leading source for high-quality Active Pharmaceutical Ingredients (APIs) and Finished Pharmaceutical Products (FPPs) in various markets across the globe. With an extensive product portfolio, our consortium of companies is dedicated to addressing and solving the most important medical needs of our time, including oncology (e.g., multiple myeloma and prostate cancer), neuroscience (e.g., schizophrenia, dementia and pain), infectious disease (e.g., HIV/AIDS, Hepatitis C and tuberculosis), and cardiovascular and metabolic diseases (e.g., diabetes). -

Lifitegrast for the Treatment of Dry Eye Disease in Adults
Expert Opinion on Pharmacotherapy ISSN: 1465-6566 (Print) 1744-7666 (Online) Journal homepage: http://www.tandfonline.com/loi/ieop20 Lifitegrast for the treatment of dry eye disease in adults Eric D. Donnenfeld, Henry D. Perry, Alanna S. Nattis & Eric D. Rosenberg To cite this article: Eric D. Donnenfeld, Henry D. Perry, Alanna S. Nattis & Eric D. Rosenberg (2017): Lifitegrast for the treatment of dry eye disease in adults, Expert Opinion on Pharmacotherapy, DOI: 10.1080/14656566.2017.1372748 To link to this article: http://dx.doi.org/10.1080/14656566.2017.1372748 Accepted author version posted online: 25 Aug 2017. Published online: 04 Sep 2017. Submit your article to this journal Article views: 11 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ieop20 Download by: [108.6.184.233] Date: 04 September 2017, At: 04:29 EXPERT OPINION ON PHARMACOTHERAPY, 2017 https://doi.org/10.1080/14656566.2017.1372748 DRUG EVALUATION Lifitegrast for the treatment of dry eye disease in adults Eric D. Donnenfelda, Henry D. Perryb, Alanna S. Nattisb and Eric D. Rosenbergc aOphthalmic Consultants of Long Island, New York University Medical Center, Garden City, NY, USA; bOphthalmic Consultants of Long Island, Nassau University Medical Center, Rockville Centre, NY, USA; cWestchester Medical Center, Valhalla, NY, USA ABSTRACT ARTICLE HISTORY Introduction: Dry eye disease (DED) is a common ocular disorder that can have a substantial burden on Received 14 June 2017 quality of life and daily activities. Lifitegrast ophthalmic solution 5.0% is the first medication approved in Accepted 24 August 2017 the US for the treatment of the signs and symptoms of DED. -

Geisinger Lewistown Hospital Published: March 25, 2019
Geisinger Lewistown Hospital Published: March 25, 2019 DESCRIPTION CHARGE Fine needle aspiration; without imaging guidance $ 607.00 Fine needle aspiration; without imaging guidance $ 286.00 Fine needle aspiration; with imaging guidance $ 2,218.00 Fine needle aspiration; with imaging guidance $ 1,691.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; first lesion $ 1,979.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; each $ 1,385.00 additional lesion (List separately in addition to code for primary procedure) Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); simple or single $ 657.00 Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); complicated or $ 986.00 multiple Incision and drainage of pilonidal cyst; simple $ 657.00 Incision and drainage of pilonidal cyst; complicated $ 3,726.00 Incision and removal of foreign body, subcutaneous tissues; simple $ 1,694.00 Incision and removal of foreign body, subcutaneous tissues; complicated $ 4,710.00 Incision and drainage of hematoma, seroma or fluid collection $ 3,470.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 1,272.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 657.00 Incision -

Re-Evaluated Data of Dissociation Constants of Alendronic, Pamidronic and Olpadronic Acids
Cent. Eur. J. Chem. • 7(1) • 2009 • 8-13 DOI: 10.2478/s11532-008-0099-z Central European Journal of Chemistry Re-evaluated data of dissociation constants of alendronic, pamidronic and olpadronic acids Invited Paper Alexander P. Boichenko1, Vadim V. Markov1, Hoan Le Kong1, Anna G. Matveeva2, Lidia P. Loginova1* 1Department of Chemical Metrology, Kharkov V.N. Karazin National University, 61077 Kharkov, Ukraine. 2A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991 Moscow, Russian Federation Received 01 April 2008; Accepted 23 October 2008 Abstract: The dissociation constants of (4-amino-1-hydroxybutylidene)bisphosphonic (alendronic) acid, (3-(dimethylamino)-1- hydroxypropylidene)bisphosphonic (olpadronic) acid and (3-amino-1-hydroxypropylidene)bisphosphonic (pamidronic) acid were obtained in aqueous solutions (0.10 М КСl) and micellar solutions of cetylpyridinium chloride (0.10 М CPC) at 25.0°C. With the exception of the third dissociation constant of alendronic acid, the dissociation constants of alendronic, olpadronic and pamidronic acids in aqueous solutions matched literature data. The possibility of sodium alendronate determination by acid-base titration by NaOH solution was theoretically grounded on the basis of re-evaluated data of alendronic acid dissociation constants. Keywords: Alendronate • Olpadronate, pamidronate, dissociation constant • Micellar media effect © Versita Warsaw and Springer-Verlag Berlin Heidelberg. 1. Introduction spectrometric [12], refractive index [13] and Sodium salts of (4-amino-1-hydroxybutylidene) conductometric [14] detection have been proposed. bisphosphonic (alendronic) acid, (3-(dimethylamino)- The anion-exchange chromatography with refractive 1-hydroxypropylidene)bisphosphonic (olpadronic) acid index detection is recommended by British and (3-amino-1-hydroxypropylidene)bisphosphonic Pharmacopoeia for the determination of sodium (pamidronic) acid are successfully used for the medical alendronate [15]. -

Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-Related Hypothalamic Neuropeptides
Georgia State University ScholarWorks @ Georgia State University Neuroscience Institute Dissertations Neuroscience Institute 8-7-2018 Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides Katherine MS West Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/neurosci_diss Recommended Citation West, Katherine MS, "Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides." Dissertation, Georgia State University, 2018. https://scholarworks.gsu.edu/neurosci_diss/36 This Dissertation is brought to you for free and open access by the Neuroscience Institute at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Neuroscience Institute Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. REGULATION OF VENTRAL TEGMENTAL AREA DOPAMINE NEURON ACTIVITY BY FEEDING-RELATED HYPOTHALAMIC NEUROPEPTIDES by KATHERINE M. S. WEST Under the Direction of Aaron Roseberry, Ph.D. ABSTRACT The prevalence of obesity has doubled worldwide since the 1980s, and having a high body mass index contributes to more deaths worldwide than being underweight. Over the past 20 years, consumption of calorie-dense foods has increased, and this is considered one of the major causes of the rapid rise in obesity. Thus, understanding the neural control of food intake is important for the development of new and effective treatments of obesity. Two important brain regions that regulate food intake are the hypothalamus and the mesocorticolimbic dopamine system. The hypothalamus is essential for the homeostatic control of feeding and body weight, while the mesocorticolimbic dopamine system, also known as the reward system, is the primary circuit for reward and motivated behavior. -

ENTRESTO (Sacubitril and Valsartan) Is a Combination of a Neprilysin Inhibitor and an Angiotensin II Receptor Blocker
HIGHLIGHTS OF PRESCRIBING INFORMATION • Adjust adult doses every 2 to 4 weeks and pediatric doses every 2 weeks These highlights do not include all the information needed to use to the target maintenance dose, as tolerated by the patient. (2.2, 2.3) ENTRESTO safely and effectively. See full prescribing information for • Reduce starting dose to half the usually recommended starting dosage for: ENTRESTO. – patients not currently taking an ACE inhibitor or ARB or previously ENTRESTO® (sacubitril and valsartan) tablets, for oral use taking a low dose of these agents (2.5) Initial U.S. Approval: 2015 – patients with severe renal impairment (2.6) – patients with moderate hepatic impairment (2.7) WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. ----------------------DOSAGE FORMS AND STRENGTHS-------------------- • When pregnancy is detected, discontinue ENTRESTO as soon as • Film-coated tablets: 24/26 mg; 49/51 mg; 97/103 mg (3) possible. (5.1) --------------------------------CONTRAINDICATIONS---------------------------- • Drugs that act directly on the renin-angiotensin system can cause • Hypersensitivity to any component. (4) injury and death to the developing fetus. (5.1) • History of angioedema related to previous ACEi or ARB therapy. (4) • ----------------------------RECENT MAJOR CHANGES------------------------- Concomitant use with ACE inhibitors. (4, 7.1) • • Indications and Usage, Adult Heart Failure (1.1) 2/2021 Concomitant use with aliskiren in patients with diabetes. (4, 7.1) ----------------------------INDICATIONS AND USAGE-------------------------- ------------------------WARNINGS AND PRECAUTIONS---------------------- ENTRESTO is indicated: • Observe for signs and symptoms of angioedema and hypotension. (5.2, 5.3) • to reduce the risk of cardiovascular death and hospitalization for heart • Monitor renal function and potassium in susceptible patients. -

Minutes of PRAC Meeting on 09-12 July 2018
6 September 2018 EMA/PRAC/576790/2018 Inspections, Human Medicines Pharmacovigilance and Committees Division Pharmacovigilance Risk Assessment Committee (PRAC) Minutes of the meeting on 09-12 July 2018 Chair: June Raine – Vice-Chair: Almath Spooner Health and safety information In accordance with the Agency’s health and safety policy, delegates were briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in the minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scope listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also change during the course of the review. Additional details on some of these procedures will be published in the PRAC meeting highlights once the procedures are finalised. Of note, the minutes are a working document primarily designed for PRAC members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006, Rev. 1). 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. -

Phvwp Class Review Bisphosphonates and Osteonecrosis of the Jaw (Alendronic Acid, Clodronic Acid, Etidronic Acid, Ibandronic
PhVWP Class Review Bisphosphonates and osteonecrosis of the jaw (alendronic acid, clodronic acid, etidronic acid, ibandronic acid, neridronic acid, pamidronic acid, risedronic acid, tiludronic acid, zoledronic acid), SPC wording agreed by the PhVWP in February 2006 Section 4.4 Pamidronic acid and zoledronic acid: “Osteonecrosis of the jaw has been reported in patients with cancer receiving treatment regimens including bisphosphonates. Many of these patients were also receiving chemotherapy and corticosteroids. The majority of reported cases have been associated with dental procedures such as tooth extraction. Many had signs of local infection including osteomyelitis. A dental examination with appropriate preventive dentistry should be considered prior to treatment with bisphosphonates in patients with concomitant risk factors (e.g. cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene). While on treatment, these patients should avoid invasive dental procedures if possible. For patients who develop osteonecrosis of the jaw while on bisphosphonate therapy, dental surgery may exacerbate the condition. For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of osteonecrosis of the jaw. Clinical judgement of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment.” Remaining bisphosphonates: “Osteonecrosis of the jaw, generally associated with tooth extraction and/or local infection (including osteomyelits) has been reported in patients with cancer receiving treatment regimens including primarily intravenously administered bisphophonates. Many of these patients were also receiving chemotherapy and corticosteroids. Osteonecrosis of the jaw has also been reported in patients with osteoporosis receiving oral bisphophonates. A dental examination with appropriate preventive dentistry should be considered prior to treatment with bisphosphonates in patients with concomitant risk factors (e.g. -
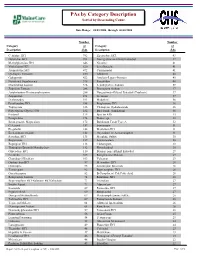
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine