Re-Evaluated Data of Dissociation Constants of Alendronic, Pamidronic and Olpadronic Acids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fosavance, INN-Alendronic Acid and Colecalciferol
EMA/175858/2015 EMEA/H/C/000619 EPAR summary for the public Fosavance alendronic acid and colecalciferol This is a summary of the European public assessment report (EPAR) for Fosavance. It explains how the Committee for Medicinal Products for Human Use (CHMP) assessed the medicine to reach its opinion in favour of granting a marketing authorisation and its recommendations on the conditions of use for Fosavance. What is Fosavance? Fosavance is a medicine that contains two active substances: alendronic acid and colecalciferol (vitamin D3). It is available as tablets (70 mg alendronic acid and 2,800 international units [IU] colecalciferol; 70 mg alendronic acid and 5,600 IU colecalciferol). What is Fosavance used for? Fosavance (containing either 2,800 or 5,600 IU colecalciferol) is used to treat osteoporosis (a disease that makes bones fragile) in women who have been through the menopause and are at risk of low vitamin D levels. Fosavance 70 mg/5,600 IU is for use in patients who are not taking vitamin D supplements. Fosavance reduces the risk of fractures (broken bones) in the spine and the hip. The medicine can only be obtained with a prescription. How is Fosavance used? The recommended dose of Fosavance is one tablet once a week. It is intended for long-term use. The patient must take the tablet with a full glass of water (but not mineral water), at least 30 minutes before any food, drink or other medicines (including antacids, calcium supplements and vitamins). To avoid irritation of the oesophagus (the tube that leads from the mouth to the stomach), the patient should not lie down until after their first food of the day, which should be at least 30 minutes after taking the tablet. -

LGM-Pharma-Regulatory-1527671011
Pipeline Products List Specialty Portfolio Updated Q2 2018 Updated Q2 2018 See below list of newly approved API’s, samples are readily available for your R&D requirements: Inhalation Ophthalmic Transdermal Sublingual Abaloparatide Defibrotide Sodium Liraglutide Rituximab Abciximab Deforolimus Lixisenatide Rivastigmine Aclidinium Bromide Azelastine HCl Agomelatine Alprazolam Abemaciclib Delafloxacin Lumacaftor Rivastigmine Hydrogen Tartrate Beclomethasone Dipropionate Azithromycin Amlodipine Aripiprazole Acalabrutinib Denosumab Matuzumab Rizatriptan Benzoate Budesonide Besifloxacin HCl Apomorphine Eletriptan HBr Aclidinium Bromide Desmopressin Acetate Meloxicam Rocuronium Bromide Adalimumab Difluprednate Memantine Hydrochloride Rolapitant Flunisolide Bimatoprost Clonidine Epinephrine Aflibercept Dinoprost Tromethamine Micafungin Romidepsin Fluticasone Furoate Brimonidine Tartrate Dextromethorphan Ergotamine Tartrate Agomelatine Dolasetron Mesylate Mitomycin C Romosozumab Fluticasone Propionate Bromfenac Sodium Diclofenac Levocetrizine DiHCl Albiglutide Donepezil Hydrochloride Mometasone Furoate Rotigotine Formoterol Fumarate Cyclosporine Donepezil Meclizine Alectinib Dorzolamide Hydrochloride Montelukast Sodium Rucaparib Iloprost Dexamethasone Valerate Estradiol Melatonin Alemtuzumab Doxercalciferol Moxifloxacin Hydrochloride Sacubitril Alirocumab Doxorubicin Hydrochloride Mycophenolate Mofetil Salmeterol Xinafoate Indacaterol Maleate Difluprednate Fingolimod Meloxicam Amphotericin B Dulaglutide Naldemedine Secukinumab Levalbuterol Dorzolamide -

Adrovance, INN-Alendronic Acid
EMA/194587/2011 EMEA/H/C/000759 EPAR summary for the public Adrovance alendronic acid / colecalciferol This is a summary of the European public assessment report (EPAR) for Adrovance. It explains how the Committee for Medicinal Products for Human Use (CHMP) assessed the medicine to reach its opinion in favour of granting a marketing authorisation and its recommendations on the conditions of use for Adrovance. What is Adrovance? Adrovance is a medicine that contains two active substances: alendronic acid and colecalciferol (vitamin D3). It is available as white tablets (capsule-shaped: 70 mg alendronic acid and 2,800 international units [IU] colecalciferol; rectangular: 70 mg alendronic acid and 5,600 IU colecalciferol). What is Adrovance used for? Adrovance (containing either 2,800 or 5,600 IU colecalciferol) is used to treat osteoporosis (a disease that makes bones fragile) in women who have been through the menopause and are at risk of low vitamin D levels. Adrovance 70 mg/5,600 IU is for use in patients who are not taking vitamin D supplements. Adrovance reduces the risk of broken bones in the spine and the hip. The medicine can only be obtained with a prescription. How is Adrovance used? The recommended dose of Adrovance is one tablet once a week. It is intended for long-term use. The patient must take the tablet with a full glass of water (but not mineral water), at least 30 minutes before any food, drink or other medicines (including antacids, calcium supplements and vitamins). To avoid irritation of the oesophagus (gullet), the patient should not lie down until after their first food of the day, which should be at least 30 minutes after taking the tablet. -

Protocol Supplementary
Optimal Pharmacological Management and Prevention of Glucocorticoid-Induced Osteoporosis (GIOP) Protocol for a Systematic Review and Network Meta-Analysis Supplementary Materials: Sample Search Strategy Supplementary 1: MEDLINE Search Strategy Database: OVID Medline Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present Line 1 exp Osteoporosis/ 2 osteoporos?s.ti,ab,kf. 3 Bone Diseases, Metabolic/ 4 osteop?eni*.ti,ab,kf. 5 Bone Diseases/ 6 exp Bone Resorption/ 7 malabsorption.ti,ab,kf. 8 Bone Density/ 9 BMD.ti,ab,kf. 10 exp Fractures, Bone/ 11 fracture*.ti,ab,kf. 12 (bone* adj2 (loss* or disease* or resorption* or densit* or content* or fragil* or mass* or demineral* or decalcif* or calcif* or strength*)).ti,ab,kf. 13 osteomalacia.ti,ab,kf. 14 or/1-13 15 exp Glucocorticoids/ 16 exp Steroids/ 17 (glucocorticoid* or steroid* or prednisone or prednisolone or hydrocortisone or cortisone or triamcinolone or dexamethasone or betamethasone or methylprednisolone).ti,ab,kf. 18 or/15-17 19 14 and 18 20 ((glucocorticoid-induced or glucosteroid-induced or corticosteroid-induced or glucocorticosteroid-induced) adj1 osteoporos?s).ti,ab,kf. 21 19 or 20 22 exp Diphosphonates/ 23 (bisphosphon* or diphosphon*).ti,ab,kf. 24 exp organophosphates/ or organophosphonates/ 25 (organophosphate* or organophosphonate*).ti,ab,kf. 26 (alendronate or alendronic acid or Fosamax or Binosto or Denfos or Fosagen or Lendrate).ti,ab,kf. 27 (Densidron or Adrovance or Alenotop or Alned or Dronat or Durost or Fixopan or Forosa or Fosval or Huesobone or Ostemax or Oseolen or Arendal or Beenos or Berlex or Fosalen or Fosmin or Fostolin or Fosavance).ti,ab,kf. -

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Catalog HPD-5E ® CREATING A HEALTHY WORLDTM Active Pharmaceutical Ingredients (APIs) Available for International Markets Human Pharmaceutical Department www.Pharmapex.net Catalog HPD-5E *Not all products referred to on this site are available in all countries and our products are subject to different regulatory requirements depending on the country of use. Consequently, certain sections of this site may be indicated as being intended only for users in specic countries. Some of the products may also be marketed under different trade names. You should not construe anything on this site as a promotion or solicitation for any product or for the use of any product that is not authorized by the laws and regulations of your country of residence. For inquiries about the availability of any specic product in your country, you may simply contact us at [email protected]. **Products currently covered by valid US Patents may be offered for R&D use in accordance with 35 USC 271(e)+A13(1). Any patent infringement and resulting liability is solely at buyer risk. ©2016, Pharmapex USA, A member of Apex Group of Companies, All Rights Reserved. Toll-Free: 1.844.PHARMAPEX Fax: + 1.619.881.0035 ACTIVE PHARMACEUTICAL [email protected] CREATING A HEALTHY WORLD™ www.Pharmapex.net INGREDIENTS About Pharmapex’s Human Pharmaceuticals Department: Pharmapex’s Human Pharmaceuticals Department (HPD) is a leading source for high-quality Active Pharmaceutical Ingredients (APIs) and Finished Pharmaceutical Products (FPPs) in various markets across the globe. With an extensive product portfolio, our consortium of companies is dedicated to addressing and solving the most important medical needs of our time, including oncology (e.g., multiple myeloma and prostate cancer), neuroscience (e.g., schizophrenia, dementia and pain), infectious disease (e.g., HIV/AIDS, Hepatitis C and tuberculosis), and cardiovascular and metabolic diseases (e.g., diabetes). -

6. Endocrine System 6.1 - Drugs Used in Diabetes Also See SIGN 116: Management of Diabetes, 2010
1 6. Endocrine System 6.1 - Drugs used in Diabetes Also see SIGN 116: Management of Diabetes, 2010 http://www.sign.ac.uk/guidelines/fulltext/116 Insulin Prescribing Guidance in Type 2 Diabetes http://www.fifeadtc.scot.nhs.uk/media/6978/insulin-prescribing-in-type-2-diabetes.pdf 6.1.1 Insulins (Type 2 Diabetes) 6.1.1.1 Short Acting Insulins 1st Choice – Insuman® Rapid (Human Insulin) – Humulin S® – Actrapid® 2nd Choice – Insulin Aspart (NovoRapid®) (Insulin Analogues) – Insulin Lispro (Humalog®) 6.1.1.2 Intermediate and Long Acting Insulins 1st Choice – Isophane Insulin (Insuman Basal®) (Human Insulin) – Isophane Insulin (Humulin I®) – Isophane Insulin (Insulatard®) 2nd Choice – Insulin Detemir (Levemir®) (Insulin Analogues) – Insulin Glargine (Lantus®) Biphasic Insulins 1st Choice – Biphasic Isophane (Human Insulin) (Insuman Comb® ‘15’, ‘25’,’50’) – Biphasic Isophane (Humulin M3®) 2nd Choice – Biphasic Aspart (Novomix® 30) (Insulin Analogues) – Biphasic Lispro (Humalog® Mix ‘25’ or ‘50’) Prescribing Points For patients with Type 1 diabetes, insulin will be initiated by a diabetes specialist with continuation of prescribing in primary care. Insulin analogues are the preferred insulins for use in Type 1 diabetes. Cartridge formulations of insulin are preferred to alternative formulations Type 2 patients who are newly prescribed insulin should usually be started on NPH isophane insulin, (e.g. Insuman Basal®, Humulin I®, Insulatard®). Long-acting recombinant human insulin analogues (e.g. Levemir®, Lantus®) offer no significant clinical advantage for most type 2 patients and are much more expensive. In terms of human insulin. The Insuman® range is currently the most cost-effective and preferred in new patients. KEY:- H – Hospital Use Only S – Specialist Initiation or Recommendation R – Restricted Use Only Fife Formulary February 2014 Last Amended June 2015 2 Patients already established on insulin should not be switched to alternative products unless recommended by a diabetes specialist. -

Phvwp Class Review Bisphosphonates and Osteonecrosis of the Jaw (Alendronic Acid, Clodronic Acid, Etidronic Acid, Ibandronic
PhVWP Class Review Bisphosphonates and osteonecrosis of the jaw (alendronic acid, clodronic acid, etidronic acid, ibandronic acid, neridronic acid, pamidronic acid, risedronic acid, tiludronic acid, zoledronic acid), SPC wording agreed by the PhVWP in February 2006 Section 4.4 Pamidronic acid and zoledronic acid: “Osteonecrosis of the jaw has been reported in patients with cancer receiving treatment regimens including bisphosphonates. Many of these patients were also receiving chemotherapy and corticosteroids. The majority of reported cases have been associated with dental procedures such as tooth extraction. Many had signs of local infection including osteomyelitis. A dental examination with appropriate preventive dentistry should be considered prior to treatment with bisphosphonates in patients with concomitant risk factors (e.g. cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene). While on treatment, these patients should avoid invasive dental procedures if possible. For patients who develop osteonecrosis of the jaw while on bisphosphonate therapy, dental surgery may exacerbate the condition. For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of osteonecrosis of the jaw. Clinical judgement of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment.” Remaining bisphosphonates: “Osteonecrosis of the jaw, generally associated with tooth extraction and/or local infection (including osteomyelits) has been reported in patients with cancer receiving treatment regimens including primarily intravenously administered bisphophonates. Many of these patients were also receiving chemotherapy and corticosteroids. Osteonecrosis of the jaw has also been reported in patients with osteoporosis receiving oral bisphophonates. A dental examination with appropriate preventive dentistry should be considered prior to treatment with bisphosphonates in patients with concomitant risk factors (e.g. -
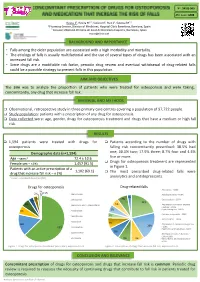
Poster EAHP 140320
Nº: 5PSQ-069 ATC code: M05 Roura J1, Rovira M1,2, Socoro N1, Ruiz S1, Sotoca JM1,2 1 Pharmacy Service, Division of Medicines, Hospital Clínic Barcelona, Barelona, Spain 2 Consorci d’Atenció Primària de Salut de Barcelona Esquerra, Barcelona, Spain [email protected] BACKGROUND AND IMPORTANCE • Falls among the older population are associated with a high morbidity and mortality. • The etiology of falls is usually multifactorial and the use of several types of drugs has been associated with an increased fall risk. • Since drugs are a modifiable risk factor, periodic drug review and eventual withdrawal of drug-related falls could be a possible strategy to prevent falls in this population. AIM AND OBJECTIVES The aim was to analyze the proportion of patients who were treated for osteoporosis and were taking, concomitantly, any drug that increase fall risk. MATERIAL AND METHODS q Observational, retrospective study in three primary care centres covering a population of 97,722 people. q Study population: patients with a prescription of any drug for osteoporosis. q Data collected were: age, gender, drugs for osteoporosis treatment and drugs that have a medium or high fall risk. RESULTS q 1,594 patients were treated with drugs for q Patients according to the number of drugs with osteoporosis falling risk concomitantly prescribed: 38.5% had Demographic data (n=1,594) one; 30.5% two; 17.9% three; 8.7% four and 4.4% five or more. Age – years* 72.4 ± 10.6 q Drugs for osteoporosis treatment are represented Female sex – n (%) 1,457 (91.5) in Figure 1. -

Best Practice & Research Clinical Rheumatology
Best Practice & Research Clinical Rheumatology 24 (2010) 489e496 Contents lists available at ScienceDirect Best Practice & Research Clinical Rheumatology journal homepage: www.elsevierhealth.com/berh 5 Novel targets in bone and cartilage Christian Beyer, Georg Schett* Department of Internal Medicine 3 and Institute for Clinical Immunology, University of Erlangen-Nuremberg, Krankenhausstrasse 12, 91054 Erlangen, Germany Keywords: The spectrum of arthritis ranges from erosive (e.g., rheumatoid bone arthritis) to ossifying disease with formation of new bone (e.g., anky- cartilage: FGF-18 losing spondylitis and osteoarthritis). The molecular basis for these hedgehog different patterns of arthritis had long been unclear. In the last few RANKL years, however, characterisation of catabolic and anabolic molecular sclerostin pathways in different forms of arthritis led to a better understanding of targets joint remodelling and revealed novel therapeutic targets. Wnt Recent findings show that catabolic and anabolic molecular pathways govern bone and cartilage remodelling in healthy and arthritic joints. The predominance of catabolic molecular pathways (e.g., receptor activator of nuclear factor-kB ligand (RANKL)/RANK and cathepsin K) causes erosive disease whereas anabolic signal- ling (e.g., Wnt and fibroblast growth factor (FGF)18) favours the formation of new bone including bony spurs and subchondral sclerosis. Other pathways may have a dual function in arthritis (e.g., hedgehog) leading to either catabolic or anabolic joint remodelling dependent on other factors. Key mediators within these signalling pathways may serve as novel targets for treating pathological remodelling of bone and cartilage in arthritis. Molecular pathways govern remodelling processes of bone and cartilage in arthritic joints. Future therapies will likely target the pathologic activity of these molecular pathways to specifically block either catabolic or anabolic joint remodelling in arthritis. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Pharmaco-Economic Study for the Prescribing of Prevention and Treatment of Osteoporosis
Technical Report 2: An analysis of the utilisation and expenditure of medicines dispensed for the prophylaxis and treatment of osteoporosis Technical report to NCAOP/HSE/DOHC By National Centre for Pharmacoeconomics An analysis of the utilisation and expenditure of medicines dispensed for the prophylaxis and treatment of osteoporosis February 2007 National Centre for Pharmacoeconomics Executive Summary 1. The number of prescriptions for the treatment and prophylaxis of osteoporosis has increased from 143,261 to 415,656 on the GMS scheme and from 52,452 to 136,547 on the DP scheme over the time period 2002 to 2005. 2. In 2005 over 60,000 patients received medications for the prophylaxis and treatment of osteoporosis on the GMS scheme with an associated expenditure of €16,093,676. 3. Approximately 80% of all patients who were dispensed drugs for the management of osteoporosis were prescribed either Alendronate (Fosamax once weekly) or Risedronate (Actonel once weekly) respectively. 4. On the DP scheme, over 27,000 patients received medications for the prophylaxis and treatment of osteoporosis in 2005 with an associated expenditure of € 6,028,925. 5. The majority of patients treated with drugs affecting bone structure were over 70 years e.g. 12,224 between 70 and 74yrs and 25,518 over 75yrs. 6. In relation to changes in treatment it was identified from the study that approximately 8% of all patients who are initiated on one treatment for osteoporosis are later switched to another therapy. 7. There was a statistically significant difference between the use of any osteoporosis medication and duration of prednisolone (dose response, chi- square test, p<0.0001). -

Fulvestrant Sponsor
CENTER FOR DRUG EVALUATION AND RESEARCH Approval Package for: APPLICATION NUMBER: 021344Orig1s026 Trade Name: Faslodex Injection Generic Name: fulvestrant Sponsor: AstraZeneca Pharmaceuticals LP Approval Date: 03/02/2016 Indications: FASLODEX is an estrogen receptor antagonist indicated for the: • Treatment of hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression following antiestrogen therapy. • Treatment of HR-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer in combination with palbociclib in women with disease progression after endocrine therapy. CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 021344Orig1s026 CONTENTS Reviews / Information Included in this NDA Review. Approval Letter X Other Action Letters Labeling X Summary Review Officer/Employee List X Office Director Memo X Cross Discipline Team Leader Review Medical Review(s) X Chemistry Review(s) X Environmental Assessment Pharmacology Review(s) Statistical Review(s) Microbiology Review(s) Clinical Pharmacology/Biopharmaceutics Review(s) X Risk Assessment and Risk Mitigation Review(s) Proprietary Name Review(s) Other Review(s) X Administrative/Correspondence Document(s) X CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 021344Orig1s026 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021344/S-026 SUPPLEMENT APPROVAL AstraZeneca Pharmaceuticals LP Attention: Elinore Mercer, PhD Director Global Regulatory Affairs One MedImmune Way Gaithersburg, MD 20878 Dear Dr. Mercer: Please refer to your Supplemental New Drug Application (sNDA) dated November 17, 2015, received November 17, 201, and your amendments, submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act (FDCA) for Faslodex® Injection (fulvestrant) Solution for Injection 250 mg/5 mL.