Preferred Drug List with Prior Authorization Criteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LGM-Pharma-Regulatory-1527671011
Pipeline Products List Specialty Portfolio Updated Q2 2018 Updated Q2 2018 See below list of newly approved API’s, samples are readily available for your R&D requirements: Inhalation Ophthalmic Transdermal Sublingual Abaloparatide Defibrotide Sodium Liraglutide Rituximab Abciximab Deforolimus Lixisenatide Rivastigmine Aclidinium Bromide Azelastine HCl Agomelatine Alprazolam Abemaciclib Delafloxacin Lumacaftor Rivastigmine Hydrogen Tartrate Beclomethasone Dipropionate Azithromycin Amlodipine Aripiprazole Acalabrutinib Denosumab Matuzumab Rizatriptan Benzoate Budesonide Besifloxacin HCl Apomorphine Eletriptan HBr Aclidinium Bromide Desmopressin Acetate Meloxicam Rocuronium Bromide Adalimumab Difluprednate Memantine Hydrochloride Rolapitant Flunisolide Bimatoprost Clonidine Epinephrine Aflibercept Dinoprost Tromethamine Micafungin Romidepsin Fluticasone Furoate Brimonidine Tartrate Dextromethorphan Ergotamine Tartrate Agomelatine Dolasetron Mesylate Mitomycin C Romosozumab Fluticasone Propionate Bromfenac Sodium Diclofenac Levocetrizine DiHCl Albiglutide Donepezil Hydrochloride Mometasone Furoate Rotigotine Formoterol Fumarate Cyclosporine Donepezil Meclizine Alectinib Dorzolamide Hydrochloride Montelukast Sodium Rucaparib Iloprost Dexamethasone Valerate Estradiol Melatonin Alemtuzumab Doxercalciferol Moxifloxacin Hydrochloride Sacubitril Alirocumab Doxorubicin Hydrochloride Mycophenolate Mofetil Salmeterol Xinafoate Indacaterol Maleate Difluprednate Fingolimod Meloxicam Amphotericin B Dulaglutide Naldemedine Secukinumab Levalbuterol Dorzolamide -

Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-Related Hypothalamic Neuropeptides
Georgia State University ScholarWorks @ Georgia State University Neuroscience Institute Dissertations Neuroscience Institute 8-7-2018 Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides Katherine MS West Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/neurosci_diss Recommended Citation West, Katherine MS, "Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides." Dissertation, Georgia State University, 2018. https://scholarworks.gsu.edu/neurosci_diss/36 This Dissertation is brought to you for free and open access by the Neuroscience Institute at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Neuroscience Institute Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. REGULATION OF VENTRAL TEGMENTAL AREA DOPAMINE NEURON ACTIVITY BY FEEDING-RELATED HYPOTHALAMIC NEUROPEPTIDES by KATHERINE M. S. WEST Under the Direction of Aaron Roseberry, Ph.D. ABSTRACT The prevalence of obesity has doubled worldwide since the 1980s, and having a high body mass index contributes to more deaths worldwide than being underweight. Over the past 20 years, consumption of calorie-dense foods has increased, and this is considered one of the major causes of the rapid rise in obesity. Thus, understanding the neural control of food intake is important for the development of new and effective treatments of obesity. Two important brain regions that regulate food intake are the hypothalamus and the mesocorticolimbic dopamine system. The hypothalamus is essential for the homeostatic control of feeding and body weight, while the mesocorticolimbic dopamine system, also known as the reward system, is the primary circuit for reward and motivated behavior. -
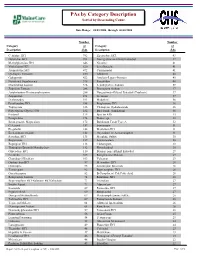
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

G Protein‐Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology (2019) 176, S21–S141 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors Stephen PH Alexander1 , Arthur Christopoulos2 , Anthony P Davenport3 , Eamonn Kelly4, Alistair Mathie5 , John A Peters6 , Emma L Veale5 ,JaneFArmstrong7 , Elena Faccenda7 ,SimonDHarding7 ,AdamJPawson7 , Joanna L Sharman7 , Christopher Southan7 , Jamie A Davies7 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Monash Institute of Pharmaceutical Sciences and Department of Pharmacology, Monash University, Parkville, Victoria 3052, Australia 3Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK 4School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 5Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 6Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 7Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. Although the Concise Guide represents approximately 400 pages, the material presented is substantially reduced compared to information and links presented on the website. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

G Protein-Coupled Receptors
Alexander, S. P. H., Christopoulos, A., Davenport, A. P., Kelly, E., Marrion, N. V., Peters, J. A., Faccenda, E., Harding, S. D., Pawson, A. J., Sharman, J. L., Southan, C., Davies, J. A. (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. British Journal of Pharmacology, 174, S17-S129. https://doi.org/10.1111/bph.13878 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1111/bph.13878 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Wiley at https://doi.org/10.1111/bph.13878 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2017/18: G protein-coupled receptors. British Journal of Pharmacology (2017) 174, S17–S129 THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors Stephen PH Alexander1, Arthur Christopoulos2, Anthony P Davenport3, Eamonn Kelly4, Neil V Marrion4, John A Peters5, Elena Faccenda6, Simon D Harding6,AdamJPawson6, Joanna L Sharman6, Christopher Southan6, Jamie A Davies6 and CGTP Collaborators 1 School of Life Sciences, -

2 12/ 35 74Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 22 March 2012 (22.03.2012) 2 12/ 35 74 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/16 (2006.01) A61K 9/51 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 9/14 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/065959 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 14 September 201 1 (14.09.201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/382,653 14 September 2010 (14.09.2010) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, NANOLOGICA AB [SE/SE]; P.O Box 8182, S-104 20 ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, Stockholm (SE). -

WO 2014/151206 Al 25 September 2014 (25.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/151206 Al 25 September 2014 (25.09.2014) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C07K 7/08 (2006.01) A61P 1/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A61K 38/10 (2006.01) A61P 29/00 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, PCT/US20 14/025207 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 13 March 2014 (13.03.2014) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (25) Filing Language: English ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/790,266 15 March 2013 (15.03.2013) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, 61/826,749 23 May 2013 (23.05.2013) US UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (71) Applicant: SYNERGY PHARMACEUTICALS INC. -

Dr. James Murrough NCT03043560 Document Date: 6/12/2019
Protocol EZOR61-01 Confidential SPONSOR: JAMES W. MURROUGH, MD INSTITUTION: ICAHN SCHOOL OF MEDICINE AT MOUNT SINAI (ISMMS) Clinical Trial Protocol DEVELOPING NEURONAL KCNQ CHANNEL MODULATORS FOR MOOD DISORDERS Protocol Number: EZOR61-01 Version #: 2.4 Version Date: 6/12/2019 Investigational Product: Ezogabine (Potiga, GlaxoSmithKline) IND Number: Exempt Development Phase: Phase IIa Sponsor: James W. Murrough, MD, PhD Assistant Professor of Psychiatry and Neuroscience Icahn School of Medicine at Mount Sinai 1 Gustave L. Levy Place, Box 1230 New York, New York 10029 Telephone: 212-585-4640 Funding Organization: National Institutes of Health (NIH)/ National Institute of Mental Health (NIMH) Principal Investigator: James W. Murrough, MD Assistant Professor of Psychiatry and Neuroscience Icahn School of Medicine at Mount Sinai 1 Gustave L. Levy Place, Box 1230 New York, New York 10029 Telephone: 212-585-4640 Fax: 212-241-3354 E-mail: [email protected] Medical Monitor: Dan V. Iosifescu, M.D., M.Sc. Associate Professor of Psychiatry NYU School of Medicine New York, NY 10016 Phone 646-754-5156 Fax 212-263-7460 [email protected] Coordinating Center: Icahn School of Medicine at Mount Sinai 1 Gustave L. Levy Place, Box 1230 New York, New York 10029 Participating site(s) Baylor College of Medicine Mood & Anxiety Disorders Program, Psychiatry PI: Sanjay Mathew, MD Version #: 2.4 Version Date: 6/12/2019 Page 1 of 66 Protocol EZOR61-01 Confidential Approval: PI or Sponsor Signature (Name and Title) Date This confidential information about an investigational product is provided for the exclusive use of investigators of this product and is subject to recall at any time. -

Neurotensin Is a Proinflammatory Neuropeptide in Colonic Inflammation
Neurotensin is a proinflammatory neuropeptide in colonic inflammation Ignazio Castagliuolo, … , Robert E. Carraway, Charalabos Pothoulakis J Clin Invest. 1999;103(6):843-849. https://doi.org/10.1172/JCI4217. Article The neuropeptide neurotensin mediates several intestinal functions, including chloride secretion, motility, and cellular growth. However, whether this peptide participates in intestinal inflammation is not known. Toxin A, an enterotoxin from Clostridium difficile, mediates pseudomembranous colitis in humans. In animal models, toxin A causes an acute inflammatory response characterized by activation of sensory neurons and intestinal nerves and immune cells of the lamina propria. Here we show that neurotensin and its receptor are elevated in the rat colonic mucosa following toxin A administration. Pretreatment of rats with the neurotensin receptor antagonist SR-48,692 inhibits toxin A–induced changes in colonic secretion, mucosal permeability, and histologic damage. Exposure of colonic explants to toxin A or neurotensin causes mast cell degranulation, which is inhibited by SR-48,692. Because substance P was previously shown to mediate mast cell activation, we examined whether substance P is involved in neurotensin-induced mast cell degranulation. Our results show that neurotensin-induced mast cell degranulation in colonic explants is inhibited by the substance P (neurokinin-1) receptor antagonist CP-96,345, indicating that colonic mast activation in response to neurotensin involves release of substance P. We conclude that neurotensin plays a key role in the pathogenesis of C. difficile–induced colonic inflammation and mast cell activation. Find the latest version: https://jci.me/4217/pdf Neurotensin is a proinflammatory neuropeptide in colonic inflammation Ignazio Castagliuolo,1 Chi-Chung Wang,1 Leyla Valenick,1 Asiya Pasha,1 Sigfus Nikulasson,2 Robert E. -

5613.Full-Text.Pdf
The Journal of Neuroscience, September 15, 1996, 16(18):5613–5620 Structure, Functional Expression, and Cerebral Localization of the Levocabastine-Sensitive Neurotensin/Neuromedin N Receptor from Mouse Brain Jean Mazella,a Jean-Marie Botto,a Eric Guillemare, Thierry Coppola, Philippe Sarret, and Jean-Pierre Vincent Institut de Pharmacologie Mole´ culaire et Cellulaire, Unite´ Propre de Recherche 411, Centre National de la Recherche Scientifique, 06560 Valbonne, France This work describes the cloning and expression of the gers an inward current. The pharmacological properties of this levocabastine-sensitive neurotensin (NT) receptor from mouse receptor correspond to those of the low-affinity, levocabastine- brain. The receptor protein comprises 417 amino acids and sensitive NT binding site described initially in membranes pre- bears the characteristics of G-protein-coupled receptors. This pared from rat and mouse brain. It is expressed maximally in new NT receptor (NTR) type is 39% homologous to, but phar- the cerebellum, hippocampus, piriform cortex, and neocortex macologically distinct from, the only other NTR cloned to date of adult mouse brain. from the rat brain and the human HT29 cell line. When the receptor is expressed in Xenopus laevis oocytes, the H1 anti- Key words: neurotensin; neuromedin N; receptor; levocabas- histaminic drug levocabastine, like NT and neuromedin N, trig- tine; cloning; low affinity; G-protein-coupled The existence of multiple receptors for the neurotensin (NT)- guinea pig striatal slices and in the turning behavior induced by related peptides was suggested initially by the description of two unilateral intrastriatal injection of NT in the mouse (Gully et al., families of NT binding sites (KD1 5 0.17 nM; KD2 5 2nM)inrat 1993).