Dr. James Murrough NCT03043560 Document Date: 6/12/2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LGM-Pharma-Regulatory-1527671011
Pipeline Products List Specialty Portfolio Updated Q2 2018 Updated Q2 2018 See below list of newly approved API’s, samples are readily available for your R&D requirements: Inhalation Ophthalmic Transdermal Sublingual Abaloparatide Defibrotide Sodium Liraglutide Rituximab Abciximab Deforolimus Lixisenatide Rivastigmine Aclidinium Bromide Azelastine HCl Agomelatine Alprazolam Abemaciclib Delafloxacin Lumacaftor Rivastigmine Hydrogen Tartrate Beclomethasone Dipropionate Azithromycin Amlodipine Aripiprazole Acalabrutinib Denosumab Matuzumab Rizatriptan Benzoate Budesonide Besifloxacin HCl Apomorphine Eletriptan HBr Aclidinium Bromide Desmopressin Acetate Meloxicam Rocuronium Bromide Adalimumab Difluprednate Memantine Hydrochloride Rolapitant Flunisolide Bimatoprost Clonidine Epinephrine Aflibercept Dinoprost Tromethamine Micafungin Romidepsin Fluticasone Furoate Brimonidine Tartrate Dextromethorphan Ergotamine Tartrate Agomelatine Dolasetron Mesylate Mitomycin C Romosozumab Fluticasone Propionate Bromfenac Sodium Diclofenac Levocetrizine DiHCl Albiglutide Donepezil Hydrochloride Mometasone Furoate Rotigotine Formoterol Fumarate Cyclosporine Donepezil Meclizine Alectinib Dorzolamide Hydrochloride Montelukast Sodium Rucaparib Iloprost Dexamethasone Valerate Estradiol Melatonin Alemtuzumab Doxercalciferol Moxifloxacin Hydrochloride Sacubitril Alirocumab Doxorubicin Hydrochloride Mycophenolate Mofetil Salmeterol Xinafoate Indacaterol Maleate Difluprednate Fingolimod Meloxicam Amphotericin B Dulaglutide Naldemedine Secukinumab Levalbuterol Dorzolamide -

(12) Patent Application Publication (10) Pub. No.: US 2012/0190743 A1 Bain Et Al
US 2012O190743A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0190743 A1 Bain et al. (43) Pub. Date: Jul. 26, 2012 (54) COMPOUNDS FOR TREATING DISORDERS Publication Classification OR DISEASES ASSOCATED WITH (51) Int. Cl NEUROKININ 2 RECEPTORACTIVITY A6II 3L/23 (2006.01) (75) Inventors: Jerald Bain, Toronto (CA); Joel CD7C 69/30 (2006.01) Sadavoy, Toronto (CA); Hao Chen, 39t. ii; C Columbia, MD (US); Xiaoyu Shen, ( .01) Columbia, MD (US) A6IPI/00 (2006.01) s A6IP 29/00 (2006.01) (73) Assignee: UNITED PARAGON A6IP II/00 (2006.01) ASSOCIATES INC., Guelph, ON A6IPI3/10 (2006.01) (CA) A6IP 5/00 (2006.01) A6IP 25/00 (2006.01) (21) Appl. No.: 13/394,067 A6IP 25/30 (2006.01) A6IP5/00 (2006.01) (22) PCT Filed: Sep. 7, 2010 A6IP3/00 (2006.01) CI2N 5/071 (2010.01) (86). PCT No.: PCT/US 10/48OO6 CD7C 69/33 (2006.01) S371 (c)(1) (52) U.S. Cl. .......................... 514/552; 554/227; 435/375 (2), (4) Date: Apr. 12, 2012 (57) ABSTRACT Related U.S. Application Data Compounds, pharmaceutical compositions and methods of (60) Provisional application No. 61/240,014, filed on Sep. treating a disorder or disease associated with neurokinin 2 4, 2009. (NK) receptor activity. Patent Application Publication Jul. 26, 2012 Sheet 1 of 12 US 2012/O190743 A1 LU 1750 15OO 1250 OOO 750 500 250 O O 20 3O 40 min SampleName: EM2OO617 Patent Application Publication Jul. 26, 2012 Sheet 2 of 12 US 2012/O190743 A1 kixto CFUgan <tro CFUgan FIG.2 Patent Application Publication Jul. -

Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-Related Hypothalamic Neuropeptides
Georgia State University ScholarWorks @ Georgia State University Neuroscience Institute Dissertations Neuroscience Institute 8-7-2018 Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides Katherine MS West Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/neurosci_diss Recommended Citation West, Katherine MS, "Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides." Dissertation, Georgia State University, 2018. https://scholarworks.gsu.edu/neurosci_diss/36 This Dissertation is brought to you for free and open access by the Neuroscience Institute at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Neuroscience Institute Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. REGULATION OF VENTRAL TEGMENTAL AREA DOPAMINE NEURON ACTIVITY BY FEEDING-RELATED HYPOTHALAMIC NEUROPEPTIDES by KATHERINE M. S. WEST Under the Direction of Aaron Roseberry, Ph.D. ABSTRACT The prevalence of obesity has doubled worldwide since the 1980s, and having a high body mass index contributes to more deaths worldwide than being underweight. Over the past 20 years, consumption of calorie-dense foods has increased, and this is considered one of the major causes of the rapid rise in obesity. Thus, understanding the neural control of food intake is important for the development of new and effective treatments of obesity. Two important brain regions that regulate food intake are the hypothalamus and the mesocorticolimbic dopamine system. The hypothalamus is essential for the homeostatic control of feeding and body weight, while the mesocorticolimbic dopamine system, also known as the reward system, is the primary circuit for reward and motivated behavior. -
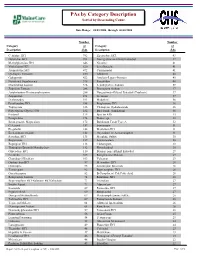
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

G Protein‐Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology (2019) 176, S21–S141 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors Stephen PH Alexander1 , Arthur Christopoulos2 , Anthony P Davenport3 , Eamonn Kelly4, Alistair Mathie5 , John A Peters6 , Emma L Veale5 ,JaneFArmstrong7 , Elena Faccenda7 ,SimonDHarding7 ,AdamJPawson7 , Joanna L Sharman7 , Christopher Southan7 , Jamie A Davies7 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Monash Institute of Pharmaceutical Sciences and Department of Pharmacology, Monash University, Parkville, Victoria 3052, Australia 3Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK 4School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 5Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 6Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 7Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. Although the Concise Guide represents approximately 400 pages, the material presented is substantially reduced compared to information and links presented on the website. -

Preferred Drug List with Prior Authorization Criteria
BUREAU FOR MEDICAL SERVICES WEST VIRGINIA MEDICAID PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA REVISED 6/1/05 Posted: 6/6/05 THERAPEUTIC PREFERRED NON-PREFERRED PA DRUG CLASS AGENTS AGENTS CRITERIA ACE INHIBITORS ACE INHIBITORS Four of the preferred agents must be tried for at least 30 days each ACEON (perindopril) ACCUPRIL (quinapril) before a non-preferred agent will be authorized unless one of the exceptions on the PA form is present. Implement 1/3/05 ALTACE (ramipril) CAPOTEN (captopril) benazepril fosinopril captopril LOTENSIN (benazepril) enalapril MONOPRIL (fosinopril) lisinopril PRINIVIL (lisinopril) MAVIK (trandolapril) quinapril moexepril UNIVASC (moexepril) VASOTEC (enalapril) ZESTRIL (lisinopril) ACE INHIBITOR/DIURETIC COMBINATIONS benazepril/HCTZ ACCURETIC (quinapril/HCTZ) captopril/HCTZ CAPOZIDE (captopril/HCTZ) enalapril/HCTZ LOTENSIN HCT (benazepril/HCTZ) lisinopril/HCTZ MONOPRIL HCT (fosinopril/HCTZ) UNIRETIC (moexepril/HCTZ) PRINZIDE (lisinopril/HCTZ) quinapril/HCTZ VASERETIC (enalapril/HCTZ) ZESTORETIC (lisinopril/HCTZ) ACE INHIBITOR/CALCIUM LOTREL (benazepril/amlodipine) LEXXEL (enalapril/felodipine) Each of the preferred agents must be tried for at least two weeks CHANNEL BLOCKER TARKA (trandolapril/verapamil) each before a non-preferred agent in that group will be authorized COMBINATIONS unless one of the exceptions on the PA form is present. Effective 7/1/05 ALZHEIMER’S AGENTS CHOLINESTERASE INHIBITORS Patients starting therapy in this class must show a documented ARICEPT (donepezil) COGNEX (tacrine) allergy to the preferred agents before a non-preferred agent will be authorized. Implement 10/1/04 EXELON (rivastigmine) REMINYL (galantamine) NMDA RECEPTOR ANTAGONIST NAMENDA (memantine) Unless otherwise specified, the listing of a particular brand or generic name includes all dosage forms of that drug. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

British Brand Name
Standardisation framework for the Maudsley staging method for treatment resistance in depression Article (Supplemental Material) Fekadu, Abebaw, Donocik, Jacek G and Cleare, Anthony J (2018) Standardisation framework for the Maudsley staging method for treatment resistance in depression. BMC Psychiatry, 18 (100). pp. 1-13. ISSN 1471-244X This version is available from Sussex Research Online: http://sro.sussex.ac.uk/id/eprint/75748/ This document is made available in accordance with publisher policies and may differ from the published version or from the version of record. If you wish to cite this item you are advised to consult the publisher’s version. Please see the URL above for details on accessing the published version. Copyright and reuse: Sussex Research Online is a digital repository of the research output of the University. Copyright and all moral rights to the version of the paper presented here belong to the individual author(s) and/or other copyright owners. To the extent reasonable and practicable, the material made available in SRO has been checked for eligibility before being made available. Copies of full text items generally can be reproduced, displayed or performed and given to third parties in any format or medium for personal research or study, educational, or not-for-profit purposes without prior permission or charge, provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. http://sro.sussex.ac.uk -

(12) Patent Application Publication (10) Pub. No.: US 2002/0102215 A1 100 Ol
US 2002O102215A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0102215 A1 Klaveness et al. (43) Pub. Date: Aug. 1, 2002 (54) DIAGNOSTIC/THERAPEUTICAGENTS (60) Provisional application No. 60/049.264, filed on Jun. 6, 1997. Provisional application No. 60/049,265, filed (75) Inventors: Jo Klaveness, Oslo (NO); Pal on Jun. 6, 1997. Provisional application No. 60/049, Rongved, Oslo (NO); Anders Hogset, 268, filed on Jun. 7, 1997. Oslo (NO); Helge Tolleshaug, Oslo (NO); Anne Naevestad, Oslo (NO); (30) Foreign Application Priority Data Halldis Hellebust, Oslo (NO); Lars Hoff, Oslo (NO); Alan Cuthbertson, Oct. 28, 1996 (GB)......................................... 9622.366.4 Oslo (NO); Dagfinn Lovhaug, Oslo Oct. 28, 1996 (GB). ... 96223672 (NO); Magne Solbakken, Oslo (NO) Oct. 28, 1996 (GB). 9622368.0 Jan. 15, 1997 (GB). ... 97OO699.3 Correspondence Address: Apr. 24, 1997 (GB). ... 9708265.5 BACON & THOMAS, PLLC Jun. 6, 1997 (GB). ... 9711842.6 4th Floor Jun. 6, 1997 (GB)......................................... 97.11846.7 625 Slaters Lane Alexandria, VA 22314-1176 (US) Publication Classification (73) Assignee: NYCOMED IMAGING AS (51) Int. Cl." .......................... A61K 49/00; A61K 48/00 (52) U.S. Cl. ............................................. 424/9.52; 514/44 (21) Appl. No.: 09/765,614 (22) Filed: Jan. 22, 2001 (57) ABSTRACT Related U.S. Application Data Targetable diagnostic and/or therapeutically active agents, (63) Continuation of application No. 08/960,054, filed on e.g. ultrasound contrast agents, having reporters comprising Oct. 29, 1997, now patented, which is a continuation gas-filled microbubbles stabilized by monolayers of film in-part of application No. 08/958,993, filed on Oct. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period
Supplementary Online Content Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naïve patients in the postoperative period. JAMA Intern Med. Published online July 11, 2016. doi:10.1001/jamainternmed.2016.3298. eTable 1. List of CPT Codes eFigure. Sample Construction Flow Chart eTable 2. List of Drug Classes eTable 3. List of Medical Comorbidities and ICD-9 Codes eTable 4. Sample Summary Characteristics, by procedure eTable 5. Risk Factors for Chronic Opioid Use Following Surgery Among Opioid Naïve Patients This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/29/2021 eTable 1. List of CPT codes CPT Codes Total Knee Arthroplasty1 27447 Total Hip Arthroplasty1 27130 Laparoscopic Cholecystectomy2 47562, 47563, 47564 Open Cholecystectomy3 47600, 47605, 47610 Laparoscopic Appendectomy4 44970, 44979 Open Appendectomy5 44950, 44960 Cesarean Section6 59510, 59514, 59515 FESS7 31237, 31240, 31254, 31255, 31256, 31267, 31276, 31287, 31288 Cataract Surgery8 66982, 66983, 66984 TURP9 52601, 52612, 52614 Simple Mastectomy10‐12 19301, 19302, 19303, 19180 © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/29/2021 eFigure. Sample Construction Flow Chart © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/29/2021 eTable 2.