Allergic Conjunctivitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LGM-Pharma-Regulatory-1527671011
Pipeline Products List Specialty Portfolio Updated Q2 2018 Updated Q2 2018 See below list of newly approved API’s, samples are readily available for your R&D requirements: Inhalation Ophthalmic Transdermal Sublingual Abaloparatide Defibrotide Sodium Liraglutide Rituximab Abciximab Deforolimus Lixisenatide Rivastigmine Aclidinium Bromide Azelastine HCl Agomelatine Alprazolam Abemaciclib Delafloxacin Lumacaftor Rivastigmine Hydrogen Tartrate Beclomethasone Dipropionate Azithromycin Amlodipine Aripiprazole Acalabrutinib Denosumab Matuzumab Rizatriptan Benzoate Budesonide Besifloxacin HCl Apomorphine Eletriptan HBr Aclidinium Bromide Desmopressin Acetate Meloxicam Rocuronium Bromide Adalimumab Difluprednate Memantine Hydrochloride Rolapitant Flunisolide Bimatoprost Clonidine Epinephrine Aflibercept Dinoprost Tromethamine Micafungin Romidepsin Fluticasone Furoate Brimonidine Tartrate Dextromethorphan Ergotamine Tartrate Agomelatine Dolasetron Mesylate Mitomycin C Romosozumab Fluticasone Propionate Bromfenac Sodium Diclofenac Levocetrizine DiHCl Albiglutide Donepezil Hydrochloride Mometasone Furoate Rotigotine Formoterol Fumarate Cyclosporine Donepezil Meclizine Alectinib Dorzolamide Hydrochloride Montelukast Sodium Rucaparib Iloprost Dexamethasone Valerate Estradiol Melatonin Alemtuzumab Doxercalciferol Moxifloxacin Hydrochloride Sacubitril Alirocumab Doxorubicin Hydrochloride Mycophenolate Mofetil Salmeterol Xinafoate Indacaterol Maleate Difluprednate Fingolimod Meloxicam Amphotericin B Dulaglutide Naldemedine Secukinumab Levalbuterol Dorzolamide -

Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

A Description of the Clinical Features of Brimonidine- Associated Uveitis Alyssa Louie Primary Care Resident, San Francisco VA
Drug-induced intraocular inflammation: A description of the clinical features of brimonidine- associated uveitis Alyssa Louie Primary Care Resident, San Francisco VA Abstract: A description of the clinical features, diagnostic work-up, and management of acute anterior uveitis caused by brimonidine, a widely used glaucoma medication. I. Case History a. Patient demographics: 74 year-old white male b. Chief complaint: eye pain, redness, irritation for last 2 weeks c. Ocular and medical history: i. Ocular history 1. Primary open angle glaucoma OU, diagnosed 8 years ago 2. Senile cataracts OU, not visually significant 3. Type 2 Diabetes without retinopathy OU 4. No prior history of uveitis ii. Medical history: Diabetes Mellitus Type 2 iii. No known drug allergies d. Medications i. Ocular: dorzolamide BID OU (1.5 years), brimonidine BID OU (11 months), travatan QHS OU (5.5 years) ii. Medical: metformin 500mg tab BID PO II. Pertinent Findings a. Clinical exam i. Visual acuities: OD 20/20-, OS 20/20- ii. Goldmann applanation tonometry: 13 mm Hg OD, 13 mm Hg OS iii. Anterior segment 1. OU: 3+ diffuse conjunctival injection 2. OU: central and inferior granulomatous keratic precipitates 3. OU: Grade 1+ cell, 1+ flare 4. OU: No synechiae or iris changes were present iv. Posterior segment 1. Optic Nerve a. OD: Cup-to-disc ratio 0.70H/V, distinct margins b. OS: Cup-to-disc ratio 0.75H/V, distinct margins 2. Posterior pole, periphery, vitreous: unremarkable OU b. Laboratory Studies i. ACE, Lysozyme, FTA-ABS, VDRL, HLA-B27, Rheumatoid Factor, ANA, PPD, Chest X- ray: all negative/unreactive III. -

MRSA Ophthalmic Infection, Part 2: Focus on Orbital Cellulitis
Clinical Update COMPREHENSIVE MRSA Ophthalmic Infection, Part 2: Focus on Orbital Cellulitis by gabrielle weiner, contributing writer interviewing preston h. blomquist, md, vikram d. durairaj, md, and david g. hwang, md rbital cellulitis is a poten- Acute MRSA Cellulitis tially sight- and life-threat- ening disease that tops the 1A 1B ophthalmology worry list. Add methicillin-resistant OStaphylococcus aureus (MRSA) to the mix of potential causative bacteria, and the level of concern rises even higher. MRSA has become a relatively prevalent cause of ophthalmic infec- tions; for example, one study showed that 89 percent of preseptal cellulitis S. aureus isolates are MRSA.1 And (1A) This 19-month-old boy presented with left periorbital edema and erythema preseptal cellulitis can rapidly develop five days after having been diagnosed in an ER with conjunctivitis and treated into the more worrisome condition of with oral and topical antibiotics. (1B) Axial CT image of the orbits with contrast orbital cellulitis if not treated promptly shows lacrimal gland abscess and globe displacement. and effectively. Moreover, the community-associ- and Hospital System in Dallas, 86 per- When to Suspect ated form of MRSA (CA-MRSA) now cent of those with preseptal cellulitis MRSA Orbital Cellulitis accounts for a larger proportion of and/or lid abscesses had CA-MRSA. Patients with orbital cellulitis com- ophthalmic cases than health care– These studies also found that preseptal monly complain of pain when moving associated MRSA (HA-MRSA). Thus, cellulitis was the most common oph- the eye, decreased vision, and limited many patients do not have the risk fac- thalmic MRSA presentation from 2000 eye movement. -

Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy Using Machine Learning
Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy using Machine Learning Kate Wang et al. Supplemental Table S1. Drugs selected by Pharmacophore-based, ML-based and DL- based search in the FDA-approved drugs database Pharmacophore WEKA TF 1-Palmitoyl-2-oleoyl-sn-glycero-3- 5-O-phosphono-alpha-D- (phospho-rac-(1-glycerol)) ribofuranosyl diphosphate Acarbose Amikacin Acetylcarnitine Acetarsol Arbutamine Acetylcholine Adenosine Aldehydo-N-Acetyl-D- Benserazide Acyclovir Glucosamine Bisoprolol Adefovir dipivoxil Alendronic acid Brivudine Alfentanil Alginic acid Cefamandole Alitretinoin alpha-Arbutin Cefdinir Azithromycin Amikacin Cefixime Balsalazide Amiloride Cefonicid Bethanechol Arbutin Ceforanide Bicalutamide Ascorbic acid calcium salt Cefotetan Calcium glubionate Auranofin Ceftibuten Cangrelor Azacitidine Ceftolozane Capecitabine Benserazide Cerivastatin Carbamoylcholine Besifloxacin Chlortetracycline Carisoprodol beta-L-fructofuranose Cilastatin Chlorobutanol Bictegravir Citicoline Cidofovir Bismuth subgallate Cladribine Clodronic acid Bleomycin Clarithromycin Colistimethate Bortezomib Clindamycin Cyclandelate Bromotheophylline Clofarabine Dexpanthenol Calcium threonate Cromoglicic acid Edoxudine Capecitabine Demeclocycline Elbasvir Capreomycin Diaminopropanol tetraacetic acid Erdosteine Carbidopa Diazolidinylurea Ethchlorvynol Carbocisteine Dibekacin Ethinamate Carboplatin Dinoprostone Famotidine Cefotetan Dipyridamole Fidaxomicin Chlormerodrin Doripenem Flavin adenine dinucleotide -

Allergies Your Amerigroup Community Care Patients May Experience a Pharmacy Claim Rejection When Prescribed Nonpreferred Products
Provider update Hot Tip: Allergies Your Amerigroup Community Care patients may experience a pharmacy claim rejection when prescribed nonpreferred products. To avoid additional steps or delays at the pharmacy, consider prescribing preferred products whenever possible. Utilization Management edits may apply to select preferred products. Coverage should be verified by reviewing the Preferred Drug List (PDL) on the Amerigroup provider website. The PDL is subject to change quarterly. Therapeutic class Preferred products Nonpreferred products Oral • Fexofenadine (generic Allegra) • Cetirizine (generic Zyrtec) antihistamines1 • Fexofenadine/ pseudoephedrine • Cetirizine/pseudoephedrine (generic Allegra-D) (generic Zyrtec D) • Loratadine (generic Claritin) • Zyrtec (cetirizine) • Loratadine/pseudoepherine • Zyrtec D (cetirizine/ (generic Claritin D) pseudoephedrine) • Clarinex (desloratadine) • Desloratadine (generic Clarinex) • Alegra (fexofenadine) • Allegra D (fexofenadine/ pseudoephedrine) • Levocetirizine (generic Xyzal) • Xyzal (levocetirizine) • Claritin (loratadine) • Claritin D (loratadine/ pseudoephedrine) Nasal steroids2 • OTC budesonide nasal spray • Flonase Sensimist (fluticasone (generic Rhinocort) furoate) • OTC Rhinocort Allergy • Flonase (fluticasone propionate) (budesonide) • Rx fluticasone propionate (generic • OTC fluticasone propionate Rx Flonase) (generic Flonase) • Mometasone furoate (generic • OTC triamcinolone acetonide Nasonex) (generic Nasacort) • Nasacort (triamcinolone acetonide) • Nasonex (mometasone furoate) • Omnaris -

Chronic Conjunctivitis
9/8/2017 Allergan Pharmaceuticals Speaker’s Bureau Bio-Tissue BioDLogics, LLC Katena/IOP Seed Biotech COA Monterey Symposium 2017 Johnson and Johnson Vision Care, Inc. Shire Pharmaceuticals Nicholas Colatrella, OD, FAAO, Dipl AAO, ABO, ABCMO Jeffrey R. Varanelli, OD, FAAO, Dipl ABO, ABCMO Text NICHOLASCOLA090 to 22333 to join Live Text Poll Nicholas Colatrella, OD, FAAO, Dipl AAO, Jeffrey Varanelli, OD, FAAO, Dipl ABO, ABO, ABCMO ABCMO Text NICHOLASCOLA090 to 22333 once to join Then text A, B, C, D, E or write in your answer Live Immediate Accurate Chronic conjunctivitis is one of the most frustrating reasons that patients present to the office (1) Time course Often times patients will seek multiple providers searching for a solution The chronicity of their symptoms is extremely frustrating to the (2) Morphology patient and treating physician alike Some conditions can seriously affect vision and create ocular morbidity (3) Localization of disease process Many of these diseases do not respond to commonly used topical antibiotics, topical steroids, artificial tears, and other treatments for external ocular disease (4) Type of discharge or exudate Our hope during this one-hour lecture is to present a process to help aid in the diagnosis of chronic conjunctivitis help you determine the most likely etiology 1 9/8/2017 Three weeks is the dividing point as it is the upper limit for cases of viral infection and most bacterial infections to resolve without treatment. Acute Conjunctivitis Conjunctivitis that has been present for less than 3 weeks -

Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-Related Hypothalamic Neuropeptides
Georgia State University ScholarWorks @ Georgia State University Neuroscience Institute Dissertations Neuroscience Institute 8-7-2018 Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides Katherine MS West Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/neurosci_diss Recommended Citation West, Katherine MS, "Regulation of Ventral Tegmental Area Dopamine Neuron Activity by Feeding-related Hypothalamic Neuropeptides." Dissertation, Georgia State University, 2018. https://scholarworks.gsu.edu/neurosci_diss/36 This Dissertation is brought to you for free and open access by the Neuroscience Institute at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Neuroscience Institute Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. REGULATION OF VENTRAL TEGMENTAL AREA DOPAMINE NEURON ACTIVITY BY FEEDING-RELATED HYPOTHALAMIC NEUROPEPTIDES by KATHERINE M. S. WEST Under the Direction of Aaron Roseberry, Ph.D. ABSTRACT The prevalence of obesity has doubled worldwide since the 1980s, and having a high body mass index contributes to more deaths worldwide than being underweight. Over the past 20 years, consumption of calorie-dense foods has increased, and this is considered one of the major causes of the rapid rise in obesity. Thus, understanding the neural control of food intake is important for the development of new and effective treatments of obesity. Two important brain regions that regulate food intake are the hypothalamus and the mesocorticolimbic dopamine system. The hypothalamus is essential for the homeostatic control of feeding and body weight, while the mesocorticolimbic dopamine system, also known as the reward system, is the primary circuit for reward and motivated behavior. -

Therapeutic Class Overview Intranasal Histamine H1-Receptor Antagonists (Antihistamines)
Therapeutic Class Overview Intranasal Histamine H1-receptor Antagonists (Antihistamines) Therapeutic Class • Overview/Summary: The four intranasal histamine-1 receptor antagonist (H1-antihistamines) products that are approved for the management of rhinitis include azelastine (Astelin®, Astepro®), olopatadine (Patanase®) and azelastine hydrochloride/fluticasone propionate (Dymista®).1-4 Allergic rhinitis, often referred to as rhinosinusitis, is a condition characterized by episodes of sneezing, rhinorrhea, nasal congestion, itchy and watery eyes, nose and palate. Other common symptoms may include cough, postnasal drip, and fatigue.5 Allergic rhinitis is also referred to in terms of the cyclical or persistent nature of symptoms. Seasonal allergic rhinitis is that which occurs at a particular time of the year; whereas, perennial allergic rhinitis describes symptoms that are present year round. Mast cell activation, histamine release, prostaglandin and leukotrienes propagation, along with other cytokine mediators (e.g., platelet activating factor, tumor necrosis factor, transforming growth factor beta, eosinophils, etc.) are known to play a direct role in the disease pathology and symptomatology.6 Allergic rhinitis may be classified by its intermittent or persistent pattern and by severity (mild or moderate to severe). Intermittent patterns involve the presence of symptoms for less than four days per week or for less than four weeks; whereas persistent patterns entail the presence of symptoms more than four days per week and for more than four weeks. Conditions associated with allergic rhinitis include: allergic conjunctivitis, sinusitis, asthma, atopic dermatitis, oral allergy syndrome, eustachian tube dysfunction, sleep disturbances, nasal obstruction leading to anosmia, and migraine headaches.5,7 Astelin® nasal spray is the only agent within the class that is available generically. -

Characteristics of Allergy in Autoimmune Thyroid Diseases Ildikó
Characteristics of allergy in autoimmune thyroid diseases Ildikó Molnár MD, PhD, EndoMed, Hungary ImmunSum, Baltimore, 2014 Relationship between allergic responses and thyroid autoimmunity IgE levels IgE deposits are present in Graves’ thyroid and orbital tissues (Werner SC et al., N Engl Med, 1972;287:421-425.; Raikow RB et al., Ophthalmol. 1990; 97:629-635.) Elevated IgE levels associated with hyperthyroid Graves’ disease (Akira S et al., J Clin Endocrinol Metab 1999; 84:3602-3605.; Takashi Y et al., J Clin Endocrinol Metab 2000; 85:2775- 2778.) Evidence of immunglobulin E autoantibodies to thyrotropin receptor (TSH rec) and thyroid peroxidase (TPO) (Metcalfe R et al., J Clin Endocrinol Metab 2002;87:1754-1761.; Gou J et al., Clin Immunol Immunopathol 1997; 82: 157-162.) Th2-derived cytokine profils Elevated serum levels of IL-5 and IL-13 cytokines. (Hidaka Y et al., Thyroid 1998; 8:235-239.; Ichiro K et al., J Clin Endocrinol Metab 2001; 86:3540-3544.) Allergic rhinitis associated frequently with Graves’ disease (Amino N et al., Thyroid 2003; 13:811-814.; Hidaka Y et al., Thyroid 1996; 6: 349-351.) Common key factors regulate the immune responses in both allergic and autoimmune conditions (Rottem M et al., Dev Immunology 2002; 9: 161-167.) ImmunSum, Baltimore, 2014 Previous results Graves’ ophthalmopathy associated with increased total IgE serum levels. Molnár I et al., Eur J Med Rev 1996; 1:543-546. Hyperthyroid Graves’ ophthalmopathy demonstrated elevated serum IL-5 levels compared to patients who had no eye signs. Molnár I , Abstract: ACT International Suppl., 2000; 2: 220. Decreased serum levels of nerve growth factor (NGF) associated with hyperthyroid Graves’ ophthalmopathy compared to those who had no eye signs. -
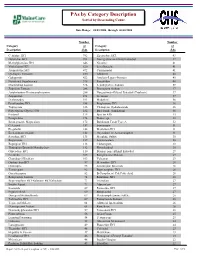
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Pediatric Pharmacology and Pathology
7/31/2017 In the next 2 hours……. Pediatric Pharmacology and Pathology . Ocular Medications and Children The content of th is COPE Accredited CE activity was prepared independently by Valerie M. Kattouf O.D. without input from members of the optometric community . Brief review of examination techniques/modifications for children The content and format of this course is presented without commercial bias and does not claim superiority of any commercial product or service . Common Presentations of Pediatric Pathology Valerie M. Kattouf O.D., F.A.A.O. Illinois College of Optometry Chief, Pediatric Binocular Vision Service Associate Professor Ocular Medications & Children Ocular Medications & Children . Pediatric systems differ in: . The rules: – drug excretion – birth 2 years old = 1/2 dose kidney is the main site of drug excretion – 2-3 years old = 2/3 dose diminished 2° renal immaturity – > 3 years old = adult dose – biotransformation liver is organ for drug metabolism Impaired 2° enzyme immaturity . If only 50 % is absorbed may be 10x maximum dosage Punctal Occlusion for 3-4 minutes ↓ systemic absorption by 40% Ocular Medications & Children Ocular Medications & Children . Systemic absorption occurs through….. Ocular Meds with strongest potential for pediatric SE : – Mucous membrane of Nasolacrimal Duct 80% of each gtt passing through NLD system is available for rapid systemic absorption by the nasal mucosa – 10 % Phenylephrine – Conjunctiva – Oropharynx – 2 % Epinephrine – Digestive system (if swallowed) Modified by variation in Gastric pH, delayed gastric emptying & intestinal mobility – 1 % Atropine – Skin (2° overflow from conjunctival sac) Greatest in infants – 2 % Cyclopentalate Blood volume of neonate 1/20 adult Therefore absorbed meds are more concentrated at this age – 1 % Prednisone 1 7/31/2017 Ocular Medications & Children Ocular Medications & Children .