Kermes Scales (Hemiptera: Kermesidae) on Oaks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kendal Kermes
K Kendal commonly known by that term in later medieval Europe: granum in Latin, grano in Italian, graine A woollen cloth.→ in French, grein in Flemish and German, and A kind of woollen cloth woven, or origi- grain in English. nally woven, in Kendal, a town in Westmorland Kermes-dyed textiles first appeared in the (now Cumbria); therefore called Kendal cloth, medieval British Isles in an urban context (prob- cloth kendalles; as an adjective it meant made of ably) in Anglo-Saxon Winchester and Anglo- Kendal cloth. The earliest references to the cloth Viking → York, but at this point kermes was date from legislation of 1390, and imply cloth of → → → confined to imported silk. Although wool the poorest→ quality (see →cloth: dimensions and textiles dyed with kermes are known from Roman weights). Gowns and hoods of Kendal are times, they do not reappear in northern Europe mentioned from c. 1443, from earlier Proceedings until the 11th century, becoming a major element in Chancery recorded→ in the reign of Elizabeth 1. in the medieval economy in the following centu- See also the naming of cloth. ries. Kermes has been discovered on ten samples Bibliography of woollen and silk textile from excavation in Kurath, H., Kuhn, S.M., Reidy, J. and Lewis, London at Swan Lane (13th century), Baynard’s R.E., ed., The Middle English Dictionary (Ann Castle (1325–50) and Custom House (1300–50). Arbor, MI: 1952–2001), s.v. Kendal. There is also a reference in the Customs Accounts of Hull, to cloth dyed with kermes coming into Elizabeth Coatsworth the port in the mid- to late 15th century. -

Garden Insects of North America: the Ultimate Guide to Backyard Bugs
© Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. CONTENTS Preface 13 Acknowledgments 15 CHAPTER one Introduction to Garden Insects and Their Relatives 16 Arthropod Growth and Metamorphosis 18 Body Parts Useful in Diagnosing Garden Insect Orders 20 Arthropods 30 Identification of Immature Stages of Common Types of Plant Injuries Caused Arthropods 21 by Insects 31 Excreted and Secreted Products Useful in Plant Pathogens Transmitted by Insects Diagnosing Garden Arthropods and Mites 39 and Slugs 28 CHAPTER Two Insects That Chew on Leaves and Needles 40 Grasshoppers 42 Giant Silkworms/Royal Moths 78 Field Crickets 46 Cecropia Moth 78 Other Crickets and Katydids 46 Other Giant Silkworms/Royal Moths 78 Common (Northern) Walkingstick 50 Slug Caterpillars/Flannel Moths and Other Related Species 50 Stinging Caterpillars 84 European Earwig 52 Tussock Moths 86 Other Earwigs 52 Whitemarked Tussock Moth 86 Related and Similar Species 86 Cockroaches 54 Gypsy Moth 90 Imported Cabbageworm 56 Other Sulfur and White Butterflies 56 Woollybears 92 Swallowtails 58 Climbing Cutworms and Armyworms 94 Parsleyworm/Black Swallowtail 58 Variegated Cutworm 94 Other Swallowtails 60 Fall Armyworm 94 Beet Armyworm 96 Brushfooted Butterflies 62 Other Climbing Cutworms and Armyworms 96 Painted Lady/Thistle Caterpillar 62 Other Brushfooted Butterflies 62 Loopers 102 Cabbage Looper 102 Hornworms and Sphinx Moths 68 Other Common Garden Loopers 102 Tomato Hornworm and Tobacco Hornworm 68 Cankerworms, Inchworms, and Other Common Hornworms 70 Spanworms 104 Fall Cankerworm 104 Prominent Moths/Notodontids 74 Other Cankerworms, Inchworms, and Walnut Caterpillar 74 Spanworms 106 Other Notodontids/Prominent Moths on Shade Trees 74 Diamondback Moth 110 Skeletonizers 110 For general queries, contact [email protected] 01 GI pp001-039.indd 5 19/07/2017 21:16 © Copyright, Princeton University Press. -

HOMOPTERA:COCCOIDEA) TÜRLERİ VE DOĞAL DÜŞMANLARI İLE ZARARLI PHENACOCCUS Aceris (SİGNORET)’İN BİYO-EKOLOJİSİ ÜZERİNDE ARAŞTIRMALAR
ANKARA ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ DOKTORA TEZİ ANKARA’DA PSEUDOCOCCIDAE (HOMOPTERA:COCCOIDEA) TÜRLERİ VE DOĞAL DÜŞMANLARI İLE ZARARLI PHENACOCCUS aceris (SİGNORET)’İN BİYO-EKOLOJİSİ ÜZERİNDE ARAŞTIRMALAR Mehmet Bora KAYDAN BİTKİ KORUMA ANABİLİM DALI ANKARA 2004 Her hakkı saklıdır 1. GİRİŞ Şehirlerdeki yeşil alanlar havadaki zehirli atıkların doğal filtresi olarak görev yapmalarının yanı sıra yaşama alanlarımızın güzel görünümü açısından da son derece önemlidir. Bu yüzden özellikle son yıllarda şehirlerde yeşillendirme çalışmaları artış göstermektedir. Şehir ekosistemlerinde süs bitkilerinin üretimi ve kullanımı, özellikle kuzey yarım kürede genel bir artış göstermekte ve gelişmiş ülkelerde şehirlerde kişi başına düşen yeşil alanın yedi m2 olması istenmektedir. Dünyadaki bu gelişmelere paralel olarak Ankara’da da yeşillendirme çalışmalarına büyük önem verilmektedir. Ankara’da kişi başına düşen yeşil alan miktarı 6.5 m2 civarındadır (Bozkurt 1992). Şehir ekosistemlerinde en büyük sorun, çevre kirliliğine ilaveten düzensiz ve bilinçsiz ilaçlamalardan dolayı doğal dengenin bozulması ve bu dengenin önemli halkalarından biri olan faydalı faunanın yok olması veya çok azalması nedeni ile zararlı fauna popülasyonunun artmasıdır. Coccoidea üst familyasının da içerisinde bulunduğu emici böceklerde bu durum daha da önemli bir boyut kazanmaktadır (Kozerveskaya, 1986). Özellikle süs bitkilerinin şehir ekosistemlerinde fizyolojik stres altında olmaları ve yeşil aksamlarının hava kirliliğinden dolayı yüksek miktarda kimyasal maddeye maruz kalmalarından dolayı coccoid enfeksiyonlarının artışına neden olmaktadır. Bu nedenle ormanlar gibi dengenin çok fazla bozulmadığı alanlarda bulunan ve nadiren ekonomik olarak önemli olan bu türler, şehir ekosistemlerinde önemli zararlılar olarak karşımıza çıkmaktadır. Aynı sorunlar çok yoğun ve sistematik ilaçlamanın yapıldığı seralarda da görülmektedir. Artan zararlı popülasyonu nedeni ile bu tip zararlıların vermiş oldukları zararlar artmakta, ekonomik kayıplara neden olmaktadır. -

The Genus Metaphycus Mercet (Hym.: Encyrtidae) of the Iranian Fauna with Description of a New Species
North-Western Journal of Zoology Vol. 6, No. 2, 2010, pp.255-261 P-ISSN: 1584-9074, E-ISSN: 1843-5629 Article No.: 061124 The genus Metaphycus Mercet (Hym.: Encyrtidae) of the Iranian fauna with description of a new species Hosseinali LOTFALIZADEH Department of Plant Protection, Agricultural Research Center of Tabriz, Azarbaijan-e Sharghi, Iran. E-mail: [email protected], [email protected] Abstract. Eleven species of Metaphycus Mercet (Hym.: Chalcidoidea, Encyrtidae) are listed for Ira- nian fauna. A new record is presented and a new species is described as M. davoodii Lotfalizadeh sp. nov. reared from Coccus hesperidum L. (Hem.: Coccidae). Illustrations and discussion of affinities of the new species with its close relatives are presented. Key words: Metaphycus davoodii sp. nov., Encyrtidae, new species, Coccidae, parasitoid, Iran. Introduction by Zeya & Hayat (1993). Species of the Nearctic region were studied by Noyes & Hanson The Encyrtidae (Hym.: Chalcidoidea) is one of (1996). Recently, a review of European species the largest families within the Chalcidoidea (Guerrieri & Noyes 2000) has contributed to with approximately 4000 nominal species the knowledge of this genus in the Palaearctic among 483 genera classified in two subfamilies region. Species of this genus are typically pri- (Noyes 2010). Fallahzadeh and Japoshvili mary endoparasitoids that live on hemipterous (2010) listed 93 species in 32 genera of encyr- families Asterolecaniidae, Coccidae, Cerococ- tids for Iranian fauna and recently Lotfalizadeh cidae, Diaspididae, Eriococcidae, Keriidae, (2010) increased this list to 102 species, corrcet- Kermesidae, Pseudococcidae and Psyllidae ing some misidentifications and adding some (Hemiptera) (Myartseva 1988, Noyes & Han- new records. The genus Metaphycus Mercet, son 1996, Guerrieri & Noyes 2000, Guerrieri with about 452 species worldwide, is a speciose 2006). -

Insects That Feed on Trees and Shrubs
INSECTS THAT FEED ON COLORADO TREES AND SHRUBS1 Whitney Cranshaw David Leatherman Boris Kondratieff Bulletin 506A TABLE OF CONTENTS DEFOLIATORS .................................................... 8 Leaf Feeding Caterpillars .............................................. 8 Cecropia Moth ................................................ 8 Polyphemus Moth ............................................. 9 Nevada Buck Moth ............................................. 9 Pandora Moth ............................................... 10 Io Moth .................................................... 10 Fall Webworm ............................................... 11 Tiger Moth ................................................. 12 American Dagger Moth ......................................... 13 Redhumped Caterpillar ......................................... 13 Achemon Sphinx ............................................. 14 Table 1. Common sphinx moths of Colorado .......................... 14 Douglas-fir Tussock Moth ....................................... 15 1. Whitney Cranshaw, Colorado State University Cooperative Extension etnomologist and associate professor, entomology; David Leatherman, entomologist, Colorado State Forest Service; Boris Kondratieff, associate professor, entomology. 8/93. ©Colorado State University Cooperative Extension. 1994. For more information, contact your county Cooperative Extension office. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, -
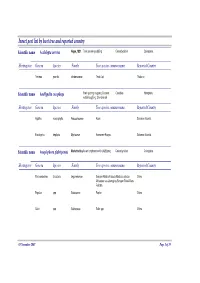
Insect Pest List by Host Tree and Reported Country
Insect pest list by host tree and reported country Scientific name Acalolepta cervina Hope, 1831 Teak canker grub|Eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Tectona grandis Verbenaceae Teak-Jati Thailand Scientific name Amblypelta cocophaga Fruit spotting bug|eng Coconut Coreidae Hemiptera nutfall bug|Eng, Chinche del Hosting tree Genera Species Family Tree species common name Reported Country Agathis macrophylla Araucariaceae Kauri Solomon Islands Eucalyptus deglupta Myrtaceae Kamarere-Bagras Solomon Islands Scientific name Anoplophora glabripennis Motschulsky Asian longhorn beetle (ALB)|eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Paraserianthes falcataria Leguminosae Sengon-Albizia-Falcata-Molucca albizia- China Moluccac sau-Jeungjing-Sengon-Batai-Mara- Falcata Populus spp. Salicaceae Poplar China Salix spp. Salicaceae Salix spp. China 05 November 2007 Page 1 of 35 Scientific name Aonidiella orientalis Newstead, Oriental scale|eng Diaspididae Homoptera 1894 Hosting tree Genera Species Family Tree species common name Reported Country Lovoa swynnertonii Meliaceae East African walnut Cameroon Azadirachta indica Meliaceae Melia indica-Neem Nigeria Scientific name Apethymus abdominalis Lepeletier, Tenthredinidae Hymenoptera 1823 Hosting tree Genera Species Family Tree species common name Reported Country Other Coniferous Other Coniferous Romania Scientific name Apriona germari Hope 1831 Long-horned beetle|eng Cerambycidae -

Historical Painting Techniques, Materials, and Studio Practice
Historical Painting Techniques, Materials, and Studio Practice PUBLICATIONS COORDINATION: Dinah Berland EDITING & PRODUCTION COORDINATION: Corinne Lightweaver EDITORIAL CONSULTATION: Jo Hill COVER DESIGN: Jackie Gallagher-Lange PRODUCTION & PRINTING: Allen Press, Inc., Lawrence, Kansas SYMPOSIUM ORGANIZERS: Erma Hermens, Art History Institute of the University of Leiden Marja Peek, Central Research Laboratory for Objects of Art and Science, Amsterdam © 1995 by The J. Paul Getty Trust All rights reserved Printed in the United States of America ISBN 0-89236-322-3 The Getty Conservation Institute is committed to the preservation of cultural heritage worldwide. The Institute seeks to advance scientiRc knowledge and professional practice and to raise public awareness of conservation. Through research, training, documentation, exchange of information, and ReId projects, the Institute addresses issues related to the conservation of museum objects and archival collections, archaeological monuments and sites, and historic bUildings and cities. The Institute is an operating program of the J. Paul Getty Trust. COVER ILLUSTRATION Gherardo Cibo, "Colchico," folio 17r of Herbarium, ca. 1570. Courtesy of the British Library. FRONTISPIECE Detail from Jan Baptiste Collaert, Color Olivi, 1566-1628. After Johannes Stradanus. Courtesy of the Rijksmuseum-Stichting, Amsterdam. Library of Congress Cataloguing-in-Publication Data Historical painting techniques, materials, and studio practice : preprints of a symposium [held at] University of Leiden, the Netherlands, 26-29 June 1995/ edited by Arie Wallert, Erma Hermens, and Marja Peek. p. cm. Includes bibliographical references. ISBN 0-89236-322-3 (pbk.) 1. Painting-Techniques-Congresses. 2. Artists' materials- -Congresses. 3. Polychromy-Congresses. I. Wallert, Arie, 1950- II. Hermens, Erma, 1958- . III. Peek, Marja, 1961- ND1500.H57 1995 751' .09-dc20 95-9805 CIP Second printing 1996 iv Contents vii Foreword viii Preface 1 Leslie A. -

May 29, 2012 Kermes Scale Kermes Scales Are Occasionally Found
Number 6 - May 29, 2012 Kermes Scale and some of the leaves die. Damage is noticed from the ground as scattered Kermes scales are occasionally found on branch tips appearing whitish and/or pin, white, bur, and other oaks in Illinois. brownish due to dead leaves and the leaf They are most common on or near the distortion revealing more leaf branch tips and appear gall-like, so they undersides. The scales feed at the base are commonly misidentified as galls. of petioles, on leaf veins, and at twig They also tend to feed in leaf axils, so crotches. The scale is rounded and about they are sometimes misidentified as one-eighth inch in diameter. Although buds. The taxonomy of kermes scale the scales are brownish, they frequently species is uncertain because early work appear blackish due to a coating of sooty relied heavily on color patterns, which mold. Sooty mold is a black fungus that we now know are variable. There are grows on the honeydew, the partially approximately 30 species of kermes that digested and concentrated plant sap occur in North America, but identification excreted by many scale insect species. is difficult. This goes beyond academic gymnastics, as different species produce Kermes scales have recently been seen crawlers at different times during the on bur oak in Illinois. These scales tend growing season. to be located on the twigs at or near leaf buds and at twig crotches near branch Control of scale insects relies on tips. They are rounded and up to one- insecticide application to the unprotected quarter inch in diameter. -

Download PDF (Inglês)
Biota Neotropica 20(4): e20201045, 2020 www.scielo.br/bn ISSN 1676-0611 (online edition) Article Anatomy of male and female reproductive organs of stink bugs pests (Pentatomidae: Heteroptera) from soybean and rice crops Vinícius Albano Araújo1* , Tito Bacca2 & Lucimar Gomes Dias3,4 1Universidade Federal do Rio de Janeiro, Instituto de Biodiversidade e Sustentabilidade, Macaé, RJ, Brasil. 2Universidad del Tolima, Facultad de Ingeniería Agronómica, Ibagué, Tolima, Colombia. 3Universidad de Caldas, Caldas, Facultad de Ciencias Exactas y Naturales, Departamento de Ciencias Biológicas, Colombia. 4Universidad de Caldas, Grupo de investigación Bionat, Caldas, Colombia. *Corresponding author: Vinícius Albano Araújo, e-mail: [email protected] ARAÚJO, V., BACCA, T., DIAS, L. Anatomy of male and female reproductive organs of stink bugs pests (Pentatomidae: Heteroptera) from soybean and rice crops. Biota Neotropica 20(4): e20201045. https://doi.org/10.1590/1676-0611-BN-2020-1045 Abstract: Pentatomidae comprises a diverse group of stink bugs widely distributed in the Neotropical region. Many species are phytophagous and cause injuries to plants, and can thus be defined as agricultural pests. In this study, the anatomy of the female and male reproductive tracts of three important agricultural pests in Colombia is described: Piezodorus guildinii Westwood, 1837 and Chinavia ubica Rolston 1983, found on soybeans, and Oebalus insularis Stål, 1872, found in rice crops. For that, light microscopy techniques were used. The anatomy of the reproductive tract of sexually mature males of the three species studied consisted of a pair of testes, vas deferens, seminal vesicles, ejaculatory bulb, an ejaculatory duct that opens into an aedeagus, and paired accessory glands. -

Statecraft and Insect Oeconomies in the Global French Enlightenment (1670-1815)
Statecraft and Insect Oeconomies in the Global French Enlightenment (1670-1815) Pierre-Etienne Stockland Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2018 © 2017 Etienne Stockland All rights reserved ABSTRACT Statecraft and Insect Oeconomies in the Global French Enlightenment (1670-1815) Pierre-Etienne Stockland Naturalists, state administrators and farmers in France and its colonies developed a myriad set of techniques over the course of the long eighteenth century to manage the circulation of useful and harmful insects. The development of normative protocols for classifying, depicting and observing insects provided a set of common tools and techniques for identifying and tracking useful and harmful insects across great distances. Administrative techniques for containing the movement of harmful insects such as quarantine, grain processing and fumigation developed at the intersection of science and statecraft, through the collaborative efforts of diplomats, state administrators, naturalists and chemical practitioners. The introduction of insectivorous animals into French colonies besieged by harmful insects was envisioned as strategy for restoring providential balance within environments suffering from human-induced disequilibria. Naturalists, administrators, and agricultural improvers also collaborated in projects to maximize the production of useful substances secreted by insects, namely silk, dyes and medicines. A study of -

Taxonomic Groups of Insects, Mites and Spiders
List Supplemental Information Content Taxonomic Groups of Insects, Mites and Spiders Pests of trees and shrubs Class Arachnida, Spiders and mites elm bark beetle, smaller European Scolytus multistriatus Order Acari, Mites and ticks elm bark beetle, native Hylurgopinus rufipes pine bark engraver, Ips pini Family Eriophyidae, Leaf vagrant, gall, erinea, rust, or pine shoot beetle, Tomicus piniperda eriophyid mites ash flower gall mite, Aceria fraxiniflora Order Hemiptera, True bugs, aphids, and scales elm eriophyid mite, Aceria parulmi Family Adelgidae, Pine and spruce aphids eriophyid mites, several species Cooley spruce gall adelgid, Adelges cooleyi hemlock rust mite, Nalepella tsugifoliae Eastern spruce gall adelgid, Adelges abietis maple spindlegall mite, Vasates aceriscrumena hemlock woolly adelgid, Adelges tsugae maple velvet erineum gall, several species pine bark adelgid, Pineus strobi Family Tarsonemidae, Cyclamen and tarsonemid mites Family Aphididae, Aphids cyclamen mite, Phytonemus pallidus balsam twig aphid, Mindarus abietinus Family Tetranychidae, Freeranging, spider mites, honeysuckle witches’ broom aphid, tetranychid mites Hyadaphis tataricae boxwood spider mite, Eurytetranychus buxi white pine aphid, Cinara strobi clover mite, Bryobia praetiosa woolly alder aphid, Paraprociphilus tessellatus European red mite, Panonychus ulmi woolly apple aphid, Eriosoma lanigerum honeylocust spider mite, Eotetranychus multidigituli Family Cercopidae, Froghoppers or spittlebugs spruce spider mite, Oligonychus ununguis spittlebugs, several -

Hymenoptera-Homoptera Associations
HOMOPTERAN ATTENDANCE BY WASPS AND ANTS: THE STOCHASTIC NATURE OF INTERACTIONS BY DEBORAH K. LETOURNEAU AND JAE C. CHOE Associations of Hymenoptera with Homoptera have intrigued ecologists and evolutionary biologists as model systems of mutual- ism. The extensive body of literature, however, tends to be skewed to the interactions between ants and homopteran trophobionts in the Aphidae or Coccoidea (e.g., Kloft et al. 1965, Nixon 1951, Way 1963, Wilson 1971). In the following account we document a web of multispecies interactions within and between trophic levels, in- volving a species of wasp, several species of ants, and two species of Homoptera. This account is unique in the literature on Hymenoptera-Homoptera associations because it (1) addresses observable interference between hymenopteran attendants, (2) reports behavioral preference by homopterans for certain hymenop- teran attendants, and (3) describes an interaction between a polis- tine wasp and an aetalionid planthopper. In addition, this study has general implications about the quality of diffuse and multiple asso- ciations between Homoptera and their honeydew foragers. MATERIALS AND METHODS Ten aggregations of feeding Aconophora ferruginea Fowler (Homoptera: Membracidae) and four of Aetalion reticulatum (L.) (Homoptera: Aetalionidae) were located in the tropical wet forest along the Quebrada Camaronal at La Sirena, Parque Nacional de Corcovado, Osa Peninsula, Costa Rica. Both species of Homoptera are common in Costa Rica, ranging from Mexico and from Costa Rica to Brazil, respectively, and possessing wide ranges of host plants (Ballou 1935, 1936, Wood 1984). They are generally sessile, mating and depositing egg masses at the feeding site (Wood 1984). Board of Environmental Studies, 407 Kerr Hall, University of California, Santa Cruz, California 95064.